UPSC Exam > UPSC Questions > Following statements are made in connection w...
Start Learning for Free
Following statements are made in connection with carbon dioxide (CO2)
1. CO2 is a poisonous gas.
2. CO2 is an acidic oxide.
3. CO2 turns limewater milky.
1. CO2 is a poisonous gas.
2. CO2 is an acidic oxide.
3. CO2 turns limewater milky.
Which of the statements given above is/are correct?
- a)1 and 2
- b)2 and 3
- c)3 only
- d)1 and 3
Correct answer is option 'B'. Can you explain this answer?
Verified Answer
Following statements are made in connection with carbon dioxide (CO2)1...
(i) CO2 is an acidic oxide. It dissolve in water formic unstable carbonic acid.
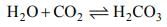
(ii) Limewater Ca(OH)2 is turned milky on passing CO2
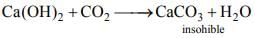
Most Upvoted Answer
Following statements are made in connection with carbon dioxide (CO2)1...
Understanding the Statements on CO2
To determine the correctness of the statements given about carbon dioxide (CO2), let’s analyze each one in detail.
Statement 1: CO2 is a poisonous gas.
- CO2 is not classified as a poisonous gas.
- While it is true that high concentrations can lead to asphyxiation by displacing oxygen, it is not toxic in the way that many other gases (like carbon monoxide) are.
Statement 2: CO2 is an acidic oxide.
- This statement is correct.
- CO2 reacts with water to form carbonic acid (H2CO3), which is a weak acid.
- Hence, it is categorized as an acidic oxide due to its ability to react with bases and form salts and water.
Statement 3: CO2 turns limewater milky.
- This statement is also correct.
- When CO2 is bubbled through limewater (a solution of calcium hydroxide), it reacts to form calcium carbonate (CaCO3), which is insoluble and appears as a milky precipitate.
Conclusion
Based on the evaluation:
- Statement 1 is incorrect.
- Statements 2 and 3 are correct.
Thus, the correct option is b) 2 and 3. This aligns with the properties and reactions of carbon dioxide in both chemistry and environmental science contexts.
To determine the correctness of the statements given about carbon dioxide (CO2), let’s analyze each one in detail.
Statement 1: CO2 is a poisonous gas.
- CO2 is not classified as a poisonous gas.
- While it is true that high concentrations can lead to asphyxiation by displacing oxygen, it is not toxic in the way that many other gases (like carbon monoxide) are.
Statement 2: CO2 is an acidic oxide.
- This statement is correct.
- CO2 reacts with water to form carbonic acid (H2CO3), which is a weak acid.
- Hence, it is categorized as an acidic oxide due to its ability to react with bases and form salts and water.
Statement 3: CO2 turns limewater milky.
- This statement is also correct.
- When CO2 is bubbled through limewater (a solution of calcium hydroxide), it reacts to form calcium carbonate (CaCO3), which is insoluble and appears as a milky precipitate.
Conclusion
Based on the evaluation:
- Statement 1 is incorrect.
- Statements 2 and 3 are correct.
Thus, the correct option is b) 2 and 3. This aligns with the properties and reactions of carbon dioxide in both chemistry and environmental science contexts.

|
Explore Courses for UPSC exam
|

|
Question Description
Following statements are made in connection with carbon dioxide (CO2)1. CO2 is a poisonous gas.2. CO2 is an acidic oxide.3. CO2 turns limewater milky.Which of the statements given above is/are correct?a)1 and 2b)2 and 3c)3 onlyd)1 and 3Correct answer is option 'B'. Can you explain this answer? for UPSC 2025 is part of UPSC preparation. The Question and answers have been prepared according to the UPSC exam syllabus. Information about Following statements are made in connection with carbon dioxide (CO2)1. CO2 is a poisonous gas.2. CO2 is an acidic oxide.3. CO2 turns limewater milky.Which of the statements given above is/are correct?a)1 and 2b)2 and 3c)3 onlyd)1 and 3Correct answer is option 'B'. Can you explain this answer? covers all topics & solutions for UPSC 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Following statements are made in connection with carbon dioxide (CO2)1. CO2 is a poisonous gas.2. CO2 is an acidic oxide.3. CO2 turns limewater milky.Which of the statements given above is/are correct?a)1 and 2b)2 and 3c)3 onlyd)1 and 3Correct answer is option 'B'. Can you explain this answer?.
Following statements are made in connection with carbon dioxide (CO2)1. CO2 is a poisonous gas.2. CO2 is an acidic oxide.3. CO2 turns limewater milky.Which of the statements given above is/are correct?a)1 and 2b)2 and 3c)3 onlyd)1 and 3Correct answer is option 'B'. Can you explain this answer? for UPSC 2025 is part of UPSC preparation. The Question and answers have been prepared according to the UPSC exam syllabus. Information about Following statements are made in connection with carbon dioxide (CO2)1. CO2 is a poisonous gas.2. CO2 is an acidic oxide.3. CO2 turns limewater milky.Which of the statements given above is/are correct?a)1 and 2b)2 and 3c)3 onlyd)1 and 3Correct answer is option 'B'. Can you explain this answer? covers all topics & solutions for UPSC 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Following statements are made in connection with carbon dioxide (CO2)1. CO2 is a poisonous gas.2. CO2 is an acidic oxide.3. CO2 turns limewater milky.Which of the statements given above is/are correct?a)1 and 2b)2 and 3c)3 onlyd)1 and 3Correct answer is option 'B'. Can you explain this answer?.
Solutions for Following statements are made in connection with carbon dioxide (CO2)1. CO2 is a poisonous gas.2. CO2 is an acidic oxide.3. CO2 turns limewater milky.Which of the statements given above is/are correct?a)1 and 2b)2 and 3c)3 onlyd)1 and 3Correct answer is option 'B'. Can you explain this answer? in English & in Hindi are available as part of our courses for UPSC.
Download more important topics, notes, lectures and mock test series for UPSC Exam by signing up for free.
Here you can find the meaning of Following statements are made in connection with carbon dioxide (CO2)1. CO2 is a poisonous gas.2. CO2 is an acidic oxide.3. CO2 turns limewater milky.Which of the statements given above is/are correct?a)1 and 2b)2 and 3c)3 onlyd)1 and 3Correct answer is option 'B'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Following statements are made in connection with carbon dioxide (CO2)1. CO2 is a poisonous gas.2. CO2 is an acidic oxide.3. CO2 turns limewater milky.Which of the statements given above is/are correct?a)1 and 2b)2 and 3c)3 onlyd)1 and 3Correct answer is option 'B'. Can you explain this answer?, a detailed solution for Following statements are made in connection with carbon dioxide (CO2)1. CO2 is a poisonous gas.2. CO2 is an acidic oxide.3. CO2 turns limewater milky.Which of the statements given above is/are correct?a)1 and 2b)2 and 3c)3 onlyd)1 and 3Correct answer is option 'B'. Can you explain this answer? has been provided alongside types of Following statements are made in connection with carbon dioxide (CO2)1. CO2 is a poisonous gas.2. CO2 is an acidic oxide.3. CO2 turns limewater milky.Which of the statements given above is/are correct?a)1 and 2b)2 and 3c)3 onlyd)1 and 3Correct answer is option 'B'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Following statements are made in connection with carbon dioxide (CO2)1. CO2 is a poisonous gas.2. CO2 is an acidic oxide.3. CO2 turns limewater milky.Which of the statements given above is/are correct?a)1 and 2b)2 and 3c)3 onlyd)1 and 3Correct answer is option 'B'. Can you explain this answer? tests, examples and also practice UPSC tests.

|
Explore Courses for UPSC exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















