Class 12 Exam > Class 12 Questions > Comprehension TypeDirection (Q, Nos. 16and 17...
Start Learning for Free
Comprehension Type
Direction (Q, Nos. 16 and 17) This section contains a paragraph, describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).
Passage
Transition metals and their compounds have paramagnetic properties due to the presence of unpaired electrons in (n - 1)d-orbitals. The paramagnetic behaviour is expressed in terms of magnetic moment which is because of the spin of the unpaired electrons (n). It is given as
Magnetic moment =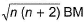
Majority of transition metal compounds are coloured both in solid state as well as in aqueous solution. This is also due to the presence of unpaired electrons in (n - 1) d-orbitals, d-orbitals splitting and d-d transition of electrons absorbing suitable visible light.
Magnetic moment =
Majority of transition metal compounds are coloured both in solid state as well as in aqueous solution. This is also due to the presence of unpaired electrons in (n - 1) d-orbitals, d-orbitals splitting and d-d transition of electrons absorbing suitable visible light.
Q.
Which of the following exhibit colour due to charge transfer phenomenon, but not due to d-d transition?
- a)
- b)
- c)
- d)All of these
Correct answer is option 'D'. Can you explain this answer?
Verified Answer
Comprehension TypeDirection (Q, Nos. 16and 17) This section contains a...
In transition metal complexes, a change in electron distribution between the metal and a ligand give rise to charge transfer bonds.
Here, charge transfer may occur from the ligand molecular orbitals to the empty or partially filled metal d-orbitals.
Here, charge transfer may occur from the ligand molecular orbitals to the empty or partially filled metal d-orbitals.
Most Upvoted Answer
Comprehension TypeDirection (Q, Nos. 16and 17) This section contains a...
D

|
Explore Courses for Class 12 exam
|

|
Similar Class 12 Doubts
Question Description
Comprehension TypeDirection (Q, Nos. 16and 17) This section contains a paragraph, describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).PassageTransition metals and their compounds have paramagnetic properties due to the presence of unpaired electrons in (n - 1)d-orbitals. The paramagnetic behaviour is expressed in terms of magnetic moment which is because of the spin of the unpaired electrons (n). It is given asMagnetic moment = Majority of transition metal compounds are coloured both in solid state as well as in aqueous solution. This is also due to the presence of unpaired electrons in (n - 1) d-orbitals, d-orbitals splitting and d-d transition of electrons absorbing suitable visible light.Q.Which of the following exhibit colour due to charge transfer phenomenon, but not due to d-d transition?a)b)c)d)All of theseCorrect answer is option 'D'. Can you explain this answer? for Class 12 2025 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about Comprehension TypeDirection (Q, Nos. 16and 17) This section contains a paragraph, describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).PassageTransition metals and their compounds have paramagnetic properties due to the presence of unpaired electrons in (n - 1)d-orbitals. The paramagnetic behaviour is expressed in terms of magnetic moment which is because of the spin of the unpaired electrons (n). It is given asMagnetic moment = Majority of transition metal compounds are coloured both in solid state as well as in aqueous solution. This is also due to the presence of unpaired electrons in (n - 1) d-orbitals, d-orbitals splitting and d-d transition of electrons absorbing suitable visible light.Q.Which of the following exhibit colour due to charge transfer phenomenon, but not due to d-d transition?a)b)c)d)All of theseCorrect answer is option 'D'. Can you explain this answer? covers all topics & solutions for Class 12 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Comprehension TypeDirection (Q, Nos. 16and 17) This section contains a paragraph, describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).PassageTransition metals and their compounds have paramagnetic properties due to the presence of unpaired electrons in (n - 1)d-orbitals. The paramagnetic behaviour is expressed in terms of magnetic moment which is because of the spin of the unpaired electrons (n). It is given asMagnetic moment = Majority of transition metal compounds are coloured both in solid state as well as in aqueous solution. This is also due to the presence of unpaired electrons in (n - 1) d-orbitals, d-orbitals splitting and d-d transition of electrons absorbing suitable visible light.Q.Which of the following exhibit colour due to charge transfer phenomenon, but not due to d-d transition?a)b)c)d)All of theseCorrect answer is option 'D'. Can you explain this answer?.
Comprehension TypeDirection (Q, Nos. 16and 17) This section contains a paragraph, describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).PassageTransition metals and their compounds have paramagnetic properties due to the presence of unpaired electrons in (n - 1)d-orbitals. The paramagnetic behaviour is expressed in terms of magnetic moment which is because of the spin of the unpaired electrons (n). It is given asMagnetic moment = Majority of transition metal compounds are coloured both in solid state as well as in aqueous solution. This is also due to the presence of unpaired electrons in (n - 1) d-orbitals, d-orbitals splitting and d-d transition of electrons absorbing suitable visible light.Q.Which of the following exhibit colour due to charge transfer phenomenon, but not due to d-d transition?a)b)c)d)All of theseCorrect answer is option 'D'. Can you explain this answer? for Class 12 2025 is part of Class 12 preparation. The Question and answers have been prepared according to the Class 12 exam syllabus. Information about Comprehension TypeDirection (Q, Nos. 16and 17) This section contains a paragraph, describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).PassageTransition metals and their compounds have paramagnetic properties due to the presence of unpaired electrons in (n - 1)d-orbitals. The paramagnetic behaviour is expressed in terms of magnetic moment which is because of the spin of the unpaired electrons (n). It is given asMagnetic moment = Majority of transition metal compounds are coloured both in solid state as well as in aqueous solution. This is also due to the presence of unpaired electrons in (n - 1) d-orbitals, d-orbitals splitting and d-d transition of electrons absorbing suitable visible light.Q.Which of the following exhibit colour due to charge transfer phenomenon, but not due to d-d transition?a)b)c)d)All of theseCorrect answer is option 'D'. Can you explain this answer? covers all topics & solutions for Class 12 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Comprehension TypeDirection (Q, Nos. 16and 17) This section contains a paragraph, describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).PassageTransition metals and their compounds have paramagnetic properties due to the presence of unpaired electrons in (n - 1)d-orbitals. The paramagnetic behaviour is expressed in terms of magnetic moment which is because of the spin of the unpaired electrons (n). It is given asMagnetic moment = Majority of transition metal compounds are coloured both in solid state as well as in aqueous solution. This is also due to the presence of unpaired electrons in (n - 1) d-orbitals, d-orbitals splitting and d-d transition of electrons absorbing suitable visible light.Q.Which of the following exhibit colour due to charge transfer phenomenon, but not due to d-d transition?a)b)c)d)All of theseCorrect answer is option 'D'. Can you explain this answer?.
Solutions for Comprehension TypeDirection (Q, Nos. 16and 17) This section contains a paragraph, describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).PassageTransition metals and their compounds have paramagnetic properties due to the presence of unpaired electrons in (n - 1)d-orbitals. The paramagnetic behaviour is expressed in terms of magnetic moment which is because of the spin of the unpaired electrons (n). It is given asMagnetic moment = Majority of transition metal compounds are coloured both in solid state as well as in aqueous solution. This is also due to the presence of unpaired electrons in (n - 1) d-orbitals, d-orbitals splitting and d-d transition of electrons absorbing suitable visible light.Q.Which of the following exhibit colour due to charge transfer phenomenon, but not due to d-d transition?a)b)c)d)All of theseCorrect answer is option 'D'. Can you explain this answer? in English & in Hindi are available as part of our courses for Class 12.
Download more important topics, notes, lectures and mock test series for Class 12 Exam by signing up for free.
Here you can find the meaning of Comprehension TypeDirection (Q, Nos. 16and 17) This section contains a paragraph, describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).PassageTransition metals and their compounds have paramagnetic properties due to the presence of unpaired electrons in (n - 1)d-orbitals. The paramagnetic behaviour is expressed in terms of magnetic moment which is because of the spin of the unpaired electrons (n). It is given asMagnetic moment = Majority of transition metal compounds are coloured both in solid state as well as in aqueous solution. This is also due to the presence of unpaired electrons in (n - 1) d-orbitals, d-orbitals splitting and d-d transition of electrons absorbing suitable visible light.Q.Which of the following exhibit colour due to charge transfer phenomenon, but not due to d-d transition?a)b)c)d)All of theseCorrect answer is option 'D'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Comprehension TypeDirection (Q, Nos. 16and 17) This section contains a paragraph, describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).PassageTransition metals and their compounds have paramagnetic properties due to the presence of unpaired electrons in (n - 1)d-orbitals. The paramagnetic behaviour is expressed in terms of magnetic moment which is because of the spin of the unpaired electrons (n). It is given asMagnetic moment = Majority of transition metal compounds are coloured both in solid state as well as in aqueous solution. This is also due to the presence of unpaired electrons in (n - 1) d-orbitals, d-orbitals splitting and d-d transition of electrons absorbing suitable visible light.Q.Which of the following exhibit colour due to charge transfer phenomenon, but not due to d-d transition?a)b)c)d)All of theseCorrect answer is option 'D'. Can you explain this answer?, a detailed solution for Comprehension TypeDirection (Q, Nos. 16and 17) This section contains a paragraph, describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).PassageTransition metals and their compounds have paramagnetic properties due to the presence of unpaired electrons in (n - 1)d-orbitals. The paramagnetic behaviour is expressed in terms of magnetic moment which is because of the spin of the unpaired electrons (n). It is given asMagnetic moment = Majority of transition metal compounds are coloured both in solid state as well as in aqueous solution. This is also due to the presence of unpaired electrons in (n - 1) d-orbitals, d-orbitals splitting and d-d transition of electrons absorbing suitable visible light.Q.Which of the following exhibit colour due to charge transfer phenomenon, but not due to d-d transition?a)b)c)d)All of theseCorrect answer is option 'D'. Can you explain this answer? has been provided alongside types of Comprehension TypeDirection (Q, Nos. 16and 17) This section contains a paragraph, describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).PassageTransition metals and their compounds have paramagnetic properties due to the presence of unpaired electrons in (n - 1)d-orbitals. The paramagnetic behaviour is expressed in terms of magnetic moment which is because of the spin of the unpaired electrons (n). It is given asMagnetic moment = Majority of transition metal compounds are coloured both in solid state as well as in aqueous solution. This is also due to the presence of unpaired electrons in (n - 1) d-orbitals, d-orbitals splitting and d-d transition of electrons absorbing suitable visible light.Q.Which of the following exhibit colour due to charge transfer phenomenon, but not due to d-d transition?a)b)c)d)All of theseCorrect answer is option 'D'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Comprehension TypeDirection (Q, Nos. 16and 17) This section contains a paragraph, describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).PassageTransition metals and their compounds have paramagnetic properties due to the presence of unpaired electrons in (n - 1)d-orbitals. The paramagnetic behaviour is expressed in terms of magnetic moment which is because of the spin of the unpaired electrons (n). It is given asMagnetic moment = Majority of transition metal compounds are coloured both in solid state as well as in aqueous solution. This is also due to the presence of unpaired electrons in (n - 1) d-orbitals, d-orbitals splitting and d-d transition of electrons absorbing suitable visible light.Q.Which of the following exhibit colour due to charge transfer phenomenon, but not due to d-d transition?a)b)c)d)All of theseCorrect answer is option 'D'. Can you explain this answer? tests, examples and also practice Class 12 tests.

|
Explore Courses for Class 12 exam
|

|
Signup to solve all Doubts
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
















