NEET Exam > NEET Questions > How many Grignard reacts with alcohols to giv...
Start Learning for Free
How many Grignard reacts with alcohols to give n-butane?
Most Upvoted Answer
How many Grignard reacts with alcohols to give n-butane?
Grignard reaction with alcohols to give n-butane
The Grignard reaction is a powerful tool in organic synthesis, and is widely used for the formation of carbon-carbon bonds. One of the applications of Grignard reagents is the synthesis of alkanes from alcohols. In this reaction, the Grignard reagent reacts with the alcohol to form an alkoxide intermediate, which subsequently undergoes a series of elimination and reduction steps to give the desired alkane. In this article, we will discuss the Grignard reaction with alcohols to give n-butane.
What is a Grignard reagent?
A Grignard reagent is an organometallic compound that contains a carbonyl group and a magnesium atom. The most common Grignard reagent is RMgX, where R is an organic group and X is a halogen (usually Cl or Br). Grignard reagents are highly reactive and can be used to form new carbon-carbon bonds.
What is an alcohol?
An alcohol is a type of organic compound that contains a hydroxyl group (-OH) attached to a carbon atom. Alcohols are commonly used as solvents, and are also important intermediates in many organic reactions.
Grignard reaction with alcohols
When a Grignard reagent reacts with an alcohol, the first step is the formation of an alkoxide intermediate. This is shown in the following equation:
RMgX + R'OH → R'OMgX + RH
In this equation, R'OMgX is the alkoxide intermediate, and RH is the alkane product. The alkoxide intermediate is formed by the reaction of the Grignard reagent with the alcohol, which results in the displacement of the hydroxyl group by the alkoxide.
Elimination and reduction steps
The alkoxide intermediate undergoes a series of elimination and reduction steps to give the desired alkane. The first step is the elimination of the magnesium atom, which results in the formation of an alkene intermediate. This is shown in the following equation:
R'OMgX → R'OH + MgX2
In this equation, MgX2 is the magnesium halide byproduct. The alkene intermediate is then reduced by the Grignard reagent to give the alkane product. This is shown in the following equation:
R'CH=CH2 + RMgX → R'CH2CH3 + MgXH
In this equation, MgXH is the magnesium halide byproduct, and R'CH2CH3 is n-butane.
Conclusion
In summary, the Grignard reaction with alcohols can be used to synthesize n-butane. The reaction involves the formation of an alkoxide intermediate, which undergoes a series of elimination and reduction steps to give the desired alkane. The Grignard reagent is a highly reactive organometallic compound that is commonly used in organic synthesis.
The Grignard reaction is a powerful tool in organic synthesis, and is widely used for the formation of carbon-carbon bonds. One of the applications of Grignard reagents is the synthesis of alkanes from alcohols. In this reaction, the Grignard reagent reacts with the alcohol to form an alkoxide intermediate, which subsequently undergoes a series of elimination and reduction steps to give the desired alkane. In this article, we will discuss the Grignard reaction with alcohols to give n-butane.
What is a Grignard reagent?
A Grignard reagent is an organometallic compound that contains a carbonyl group and a magnesium atom. The most common Grignard reagent is RMgX, where R is an organic group and X is a halogen (usually Cl or Br). Grignard reagents are highly reactive and can be used to form new carbon-carbon bonds.
What is an alcohol?
An alcohol is a type of organic compound that contains a hydroxyl group (-OH) attached to a carbon atom. Alcohols are commonly used as solvents, and are also important intermediates in many organic reactions.
Grignard reaction with alcohols
When a Grignard reagent reacts with an alcohol, the first step is the formation of an alkoxide intermediate. This is shown in the following equation:
RMgX + R'OH → R'OMgX + RH
In this equation, R'OMgX is the alkoxide intermediate, and RH is the alkane product. The alkoxide intermediate is formed by the reaction of the Grignard reagent with the alcohol, which results in the displacement of the hydroxyl group by the alkoxide.
Elimination and reduction steps
The alkoxide intermediate undergoes a series of elimination and reduction steps to give the desired alkane. The first step is the elimination of the magnesium atom, which results in the formation of an alkene intermediate. This is shown in the following equation:
R'OMgX → R'OH + MgX2
In this equation, MgX2 is the magnesium halide byproduct. The alkene intermediate is then reduced by the Grignard reagent to give the alkane product. This is shown in the following equation:
R'CH=CH2 + RMgX → R'CH2CH3 + MgXH
In this equation, MgXH is the magnesium halide byproduct, and R'CH2CH3 is n-butane.
Conclusion
In summary, the Grignard reaction with alcohols can be used to synthesize n-butane. The reaction involves the formation of an alkoxide intermediate, which undergoes a series of elimination and reduction steps to give the desired alkane. The Grignard reagent is a highly reactive organometallic compound that is commonly used in organic synthesis.
Community Answer
How many Grignard reacts with alcohols to give n-butane?
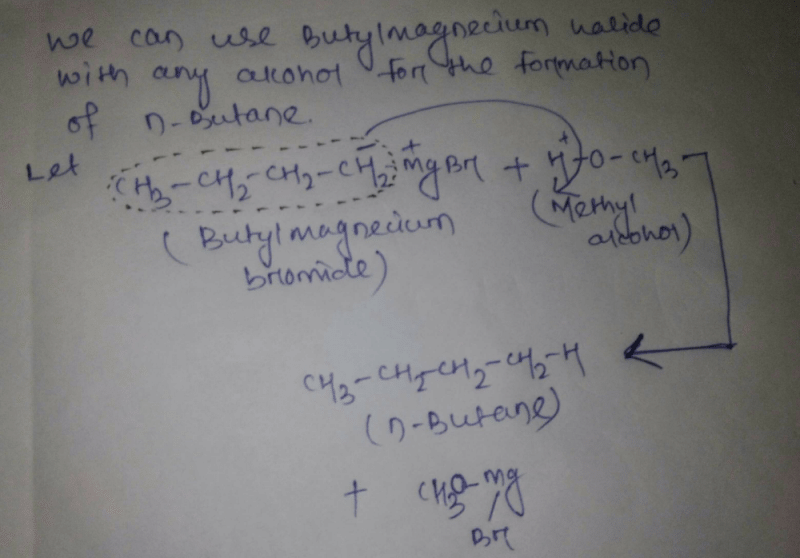

|
Explore Courses for NEET exam
|

|
Similar NEET Doubts
How many Grignard reacts with alcohols to give n-butane?
Question Description
How many Grignard reacts with alcohols to give n-butane? for NEET 2025 is part of NEET preparation. The Question and answers have been prepared according to the NEET exam syllabus. Information about How many Grignard reacts with alcohols to give n-butane? covers all topics & solutions for NEET 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for How many Grignard reacts with alcohols to give n-butane?.
How many Grignard reacts with alcohols to give n-butane? for NEET 2025 is part of NEET preparation. The Question and answers have been prepared according to the NEET exam syllabus. Information about How many Grignard reacts with alcohols to give n-butane? covers all topics & solutions for NEET 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for How many Grignard reacts with alcohols to give n-butane?.
Solutions for How many Grignard reacts with alcohols to give n-butane? in English & in Hindi are available as part of our courses for NEET.
Download more important topics, notes, lectures and mock test series for NEET Exam by signing up for free.
Here you can find the meaning of How many Grignard reacts with alcohols to give n-butane? defined & explained in the simplest way possible. Besides giving the explanation of
How many Grignard reacts with alcohols to give n-butane?, a detailed solution for How many Grignard reacts with alcohols to give n-butane? has been provided alongside types of How many Grignard reacts with alcohols to give n-butane? theory, EduRev gives you an
ample number of questions to practice How many Grignard reacts with alcohols to give n-butane? tests, examples and also practice NEET tests.

|
Explore Courses for NEET exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
























