JEE Exam > JEE Questions > Geometrical shapes of the complexes formed by...
Start Learning for Free
Geometrical shapes of the complexes formed by the reaction of Ni2+ with Cl– , CN– and H2O, respectively, are (2011)
- a)octahedral, tetrahedral and square planar
- b)tetrahedral, square planar and octahedral
- c)square planar, tetrahedral and octahedral
- d)octahedral, square planar and octahedral
Correct answer is option 'B'. Can you explain this answer?
Verified Answer
Geometrical shapes of the complexes formed by the reaction of Ni2+ wit...
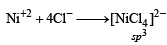
[NiCl4]2–. = 3d8 configuration with nickel in +2 oxidation state, Cl– being weak field ligand does not compel for pairing of electrons.
So,
[NiCl4]2–
So,
[NiCl4]2–
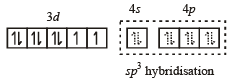
Hence, complex has tetrahedral geometry
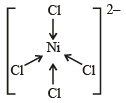
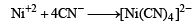
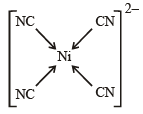
[Ni(CN)4]2– = 3d8 configuration with nickel in + 2 oxidation state, CN– being strong field ligand compels for pairing of electrons.
So,
So,
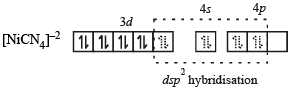
Hence, complex has square planar geometry.
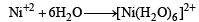
[Ni(H2O)6] = 3d8 configuration with nickel in +2 oxidation state. As with 3d8 configuration two dorbitals are not available for d2sp3 hybridisation. So, hybridisation of Ni (II) is sp3d2 and Ni (II) with six co-ordination will have octahedral geometry.
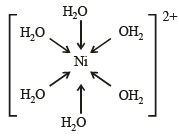
Note : With water as ligand, Ni (II) forms octahedral complexes.
Most Upvoted Answer
Geometrical shapes of the complexes formed by the reaction of Ni2+ wit...
The complex formed by the reaction of Ni2+ with Cl- can have different geometrical shapes depending on the number of ligands surrounding the central metal ion. Here are some possible geometries:
1. Square planar: In this geometry, the Ni2+ ion is surrounded by four ligands in a flat square arrangement. Each Cl- ion acts as a ligand, forming coordination bonds with the metal ion.
2. Tetrahedral: In this geometry, the Ni2+ ion is surrounded by four ligands in a tetrahedral arrangement. Each Cl- ion acts as a ligand, forming coordination bonds with the metal ion.
3. Octahedral: In this geometry, the Ni2+ ion is surrounded by six ligands in an octahedral arrangement. Each Cl- ion acts as a ligand, forming coordination bonds with the metal ion. This geometry can be achieved by having two Cl- ions in a cis arrangement and two Cl- ions in a trans arrangement around the Ni2+ ion.
These are just a few possible geometries, and the actual geometry of the complex formed by Ni2+ with Cl- will depend on factors such as the nature of the ligands and the coordination number of the metal ion.
1. Square planar: In this geometry, the Ni2+ ion is surrounded by four ligands in a flat square arrangement. Each Cl- ion acts as a ligand, forming coordination bonds with the metal ion.
2. Tetrahedral: In this geometry, the Ni2+ ion is surrounded by four ligands in a tetrahedral arrangement. Each Cl- ion acts as a ligand, forming coordination bonds with the metal ion.
3. Octahedral: In this geometry, the Ni2+ ion is surrounded by six ligands in an octahedral arrangement. Each Cl- ion acts as a ligand, forming coordination bonds with the metal ion. This geometry can be achieved by having two Cl- ions in a cis arrangement and two Cl- ions in a trans arrangement around the Ni2+ ion.
These are just a few possible geometries, and the actual geometry of the complex formed by Ni2+ with Cl- will depend on factors such as the nature of the ligands and the coordination number of the metal ion.

|
Explore Courses for JEE exam
|

|
Similar JEE Doubts
Question Description
Geometrical shapes of the complexes formed by the reaction of Ni2+ with Cl– , CN– and H2O, respectively, are (2011)a)octahedral, tetrahedral and square planarb)tetrahedral, square planar and octahedralc)square planar, tetrahedral and octahedrald)octahedral, square planar and octahedralCorrect answer is option 'B'. Can you explain this answer? for JEE 2025 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about Geometrical shapes of the complexes formed by the reaction of Ni2+ with Cl– , CN– and H2O, respectively, are (2011)a)octahedral, tetrahedral and square planarb)tetrahedral, square planar and octahedralc)square planar, tetrahedral and octahedrald)octahedral, square planar and octahedralCorrect answer is option 'B'. Can you explain this answer? covers all topics & solutions for JEE 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Geometrical shapes of the complexes formed by the reaction of Ni2+ with Cl– , CN– and H2O, respectively, are (2011)a)octahedral, tetrahedral and square planarb)tetrahedral, square planar and octahedralc)square planar, tetrahedral and octahedrald)octahedral, square planar and octahedralCorrect answer is option 'B'. Can you explain this answer?.
Geometrical shapes of the complexes formed by the reaction of Ni2+ with Cl– , CN– and H2O, respectively, are (2011)a)octahedral, tetrahedral and square planarb)tetrahedral, square planar and octahedralc)square planar, tetrahedral and octahedrald)octahedral, square planar and octahedralCorrect answer is option 'B'. Can you explain this answer? for JEE 2025 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about Geometrical shapes of the complexes formed by the reaction of Ni2+ with Cl– , CN– and H2O, respectively, are (2011)a)octahedral, tetrahedral and square planarb)tetrahedral, square planar and octahedralc)square planar, tetrahedral and octahedrald)octahedral, square planar and octahedralCorrect answer is option 'B'. Can you explain this answer? covers all topics & solutions for JEE 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Geometrical shapes of the complexes formed by the reaction of Ni2+ with Cl– , CN– and H2O, respectively, are (2011)a)octahedral, tetrahedral and square planarb)tetrahedral, square planar and octahedralc)square planar, tetrahedral and octahedrald)octahedral, square planar and octahedralCorrect answer is option 'B'. Can you explain this answer?.
Solutions for Geometrical shapes of the complexes formed by the reaction of Ni2+ with Cl– , CN– and H2O, respectively, are (2011)a)octahedral, tetrahedral and square planarb)tetrahedral, square planar and octahedralc)square planar, tetrahedral and octahedrald)octahedral, square planar and octahedralCorrect answer is option 'B'. Can you explain this answer? in English & in Hindi are available as part of our courses for JEE.
Download more important topics, notes, lectures and mock test series for JEE Exam by signing up for free.
Here you can find the meaning of Geometrical shapes of the complexes formed by the reaction of Ni2+ with Cl– , CN– and H2O, respectively, are (2011)a)octahedral, tetrahedral and square planarb)tetrahedral, square planar and octahedralc)square planar, tetrahedral and octahedrald)octahedral, square planar and octahedralCorrect answer is option 'B'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Geometrical shapes of the complexes formed by the reaction of Ni2+ with Cl– , CN– and H2O, respectively, are (2011)a)octahedral, tetrahedral and square planarb)tetrahedral, square planar and octahedralc)square planar, tetrahedral and octahedrald)octahedral, square planar and octahedralCorrect answer is option 'B'. Can you explain this answer?, a detailed solution for Geometrical shapes of the complexes formed by the reaction of Ni2+ with Cl– , CN– and H2O, respectively, are (2011)a)octahedral, tetrahedral and square planarb)tetrahedral, square planar and octahedralc)square planar, tetrahedral and octahedrald)octahedral, square planar and octahedralCorrect answer is option 'B'. Can you explain this answer? has been provided alongside types of Geometrical shapes of the complexes formed by the reaction of Ni2+ with Cl– , CN– and H2O, respectively, are (2011)a)octahedral, tetrahedral and square planarb)tetrahedral, square planar and octahedralc)square planar, tetrahedral and octahedrald)octahedral, square planar and octahedralCorrect answer is option 'B'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Geometrical shapes of the complexes formed by the reaction of Ni2+ with Cl– , CN– and H2O, respectively, are (2011)a)octahedral, tetrahedral and square planarb)tetrahedral, square planar and octahedralc)square planar, tetrahedral and octahedrald)octahedral, square planar and octahedralCorrect answer is option 'B'. Can you explain this answer? tests, examples and also practice JEE tests.

|
Explore Courses for JEE exam
|

|
Signup to solve all Doubts
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.






















