JEE Exam > JEE Questions > Geometrical shapes of the complexes formed b...
Start Learning for Free
Geometrical shapes of the complexes formed by the reaction of Ni2+ with Cl-, CN- and H2O, respectively, are
- a)octahedral, tetrahedral and square planar
- b)tetrahedral, square planar and octahedral
- c)square planar, tetrahedral and octahedral
- d)octahedral, square planar and octahedral
Correct answer is option 'B'. Can you explain this answer?
Most Upvoted Answer
Geometrical shapes of the complexes formed by the reaction of Ni2+ wi...
Ni2+ + 4Cl- → [NiCl4]2- - tetrahedral due to sp3 hybridisation.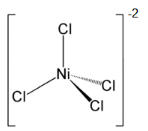
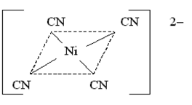

Ni2+ + 4CN- → [Ni(CN)4]2- - dsp2 hybridisation, it is square planar.

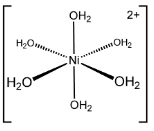
Ni2+ + 6H2O → [Ni(H2O)6]2+ - sp3d2 hybridisation, it is octahedral.

Community Answer
Geometrical shapes of the complexes formed by the reaction of Ni2+ wi...
Introduction
The geometry of metal complexes depends on the nature of the ligands and the coordination number of the metal ion. Nickel (Ni2+) can form various complexes with different geometries based on the ligands involved.
Geometries of Nickel Complexes
- Ni2+ with Cl-:
- Geometry: Tetrahedral
- Explanation: Chloride ions are weak field ligands, and they can lead to the formation of tetrahedral complexes with Ni2+. This is especially true when Ni2+ is in a low oxidation state and has a tendency to coordinate in tetrahedral geometry.
- Ni2+ with CN-:
- Geometry: Square Planar
- Explanation: Cyanide is a strong field ligand and has a strong tendency to cause pairing of electrons in the d-orbitals of Ni2+. This results in a square planar geometry, which is common for d8 metal ions like Ni2+ in this environment.
- Ni2+ with H2O:
- Geometry: Octahedral
- Explanation: Water is a neutral ligand and, when coordinated with Ni2+, typically forms octahedral complexes. This is due to its ability to donate lone pairs and the preference of Ni2+ for coordination numbers of six.
Conclusion
Thus, when Ni2+ forms complexes with Cl-, CN-, and H2O, the resulting geometries are:
- Ni2+ + Cl-: Tetrahedral
- Ni2+ + CN-: Square Planar
- Ni2+ + H2O: Octahedral
Hence, the correct answer is option 'B': Tetrahedral, Square Planar, and Octahedral.
The geometry of metal complexes depends on the nature of the ligands and the coordination number of the metal ion. Nickel (Ni2+) can form various complexes with different geometries based on the ligands involved.
Geometries of Nickel Complexes
- Ni2+ with Cl-:
- Geometry: Tetrahedral
- Explanation: Chloride ions are weak field ligands, and they can lead to the formation of tetrahedral complexes with Ni2+. This is especially true when Ni2+ is in a low oxidation state and has a tendency to coordinate in tetrahedral geometry.
- Ni2+ with CN-:
- Geometry: Square Planar
- Explanation: Cyanide is a strong field ligand and has a strong tendency to cause pairing of electrons in the d-orbitals of Ni2+. This results in a square planar geometry, which is common for d8 metal ions like Ni2+ in this environment.
- Ni2+ with H2O:
- Geometry: Octahedral
- Explanation: Water is a neutral ligand and, when coordinated with Ni2+, typically forms octahedral complexes. This is due to its ability to donate lone pairs and the preference of Ni2+ for coordination numbers of six.
Conclusion
Thus, when Ni2+ forms complexes with Cl-, CN-, and H2O, the resulting geometries are:
- Ni2+ + Cl-: Tetrahedral
- Ni2+ + CN-: Square Planar
- Ni2+ + H2O: Octahedral
Hence, the correct answer is option 'B': Tetrahedral, Square Planar, and Octahedral.

|
Explore Courses for JEE exam
|

|
Similar JEE Doubts
Question Description
Geometrical shapes of the complexes formed by the reaction of Ni2+ with Cl-, CN- and H2O, respectively, area)octahedral, tetrahedral and square planarb)tetrahedral, square planar and octahedralc)square planar, tetrahedral and octahedrald)octahedral, square planar and octahedralCorrect answer is option 'B'. Can you explain this answer? for JEE 2025 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about Geometrical shapes of the complexes formed by the reaction of Ni2+ with Cl-, CN- and H2O, respectively, area)octahedral, tetrahedral and square planarb)tetrahedral, square planar and octahedralc)square planar, tetrahedral and octahedrald)octahedral, square planar and octahedralCorrect answer is option 'B'. Can you explain this answer? covers all topics & solutions for JEE 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Geometrical shapes of the complexes formed by the reaction of Ni2+ with Cl-, CN- and H2O, respectively, area)octahedral, tetrahedral and square planarb)tetrahedral, square planar and octahedralc)square planar, tetrahedral and octahedrald)octahedral, square planar and octahedralCorrect answer is option 'B'. Can you explain this answer?.
Geometrical shapes of the complexes formed by the reaction of Ni2+ with Cl-, CN- and H2O, respectively, area)octahedral, tetrahedral and square planarb)tetrahedral, square planar and octahedralc)square planar, tetrahedral and octahedrald)octahedral, square planar and octahedralCorrect answer is option 'B'. Can you explain this answer? for JEE 2025 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about Geometrical shapes of the complexes formed by the reaction of Ni2+ with Cl-, CN- and H2O, respectively, area)octahedral, tetrahedral and square planarb)tetrahedral, square planar and octahedralc)square planar, tetrahedral and octahedrald)octahedral, square planar and octahedralCorrect answer is option 'B'. Can you explain this answer? covers all topics & solutions for JEE 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Geometrical shapes of the complexes formed by the reaction of Ni2+ with Cl-, CN- and H2O, respectively, area)octahedral, tetrahedral and square planarb)tetrahedral, square planar and octahedralc)square planar, tetrahedral and octahedrald)octahedral, square planar and octahedralCorrect answer is option 'B'. Can you explain this answer?.
Solutions for Geometrical shapes of the complexes formed by the reaction of Ni2+ with Cl-, CN- and H2O, respectively, area)octahedral, tetrahedral and square planarb)tetrahedral, square planar and octahedralc)square planar, tetrahedral and octahedrald)octahedral, square planar and octahedralCorrect answer is option 'B'. Can you explain this answer? in English & in Hindi are available as part of our courses for JEE.
Download more important topics, notes, lectures and mock test series for JEE Exam by signing up for free.
Here you can find the meaning of Geometrical shapes of the complexes formed by the reaction of Ni2+ with Cl-, CN- and H2O, respectively, area)octahedral, tetrahedral and square planarb)tetrahedral, square planar and octahedralc)square planar, tetrahedral and octahedrald)octahedral, square planar and octahedralCorrect answer is option 'B'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Geometrical shapes of the complexes formed by the reaction of Ni2+ with Cl-, CN- and H2O, respectively, area)octahedral, tetrahedral and square planarb)tetrahedral, square planar and octahedralc)square planar, tetrahedral and octahedrald)octahedral, square planar and octahedralCorrect answer is option 'B'. Can you explain this answer?, a detailed solution for Geometrical shapes of the complexes formed by the reaction of Ni2+ with Cl-, CN- and H2O, respectively, area)octahedral, tetrahedral and square planarb)tetrahedral, square planar and octahedralc)square planar, tetrahedral and octahedrald)octahedral, square planar and octahedralCorrect answer is option 'B'. Can you explain this answer? has been provided alongside types of Geometrical shapes of the complexes formed by the reaction of Ni2+ with Cl-, CN- and H2O, respectively, area)octahedral, tetrahedral and square planarb)tetrahedral, square planar and octahedralc)square planar, tetrahedral and octahedrald)octahedral, square planar and octahedralCorrect answer is option 'B'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Geometrical shapes of the complexes formed by the reaction of Ni2+ with Cl-, CN- and H2O, respectively, area)octahedral, tetrahedral and square planarb)tetrahedral, square planar and octahedralc)square planar, tetrahedral and octahedrald)octahedral, square planar and octahedralCorrect answer is option 'B'. Can you explain this answer? tests, examples and also practice JEE tests.

|
Explore Courses for JEE exam
|

|
Signup to solve all Doubts
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.



























