Class 10 Exam > Class 10 Questions > which of the following have highest resonance...
Start Learning for Free
which of the following have highest resonance energy? a)pyrrole b)furan c)pyridine. correct option is c)pyridine. can you explain wgy?(why*?)
? Related: Doubts. Please write solutions
Most Upvoted Answer
which of the following have highest resonance energy? ...
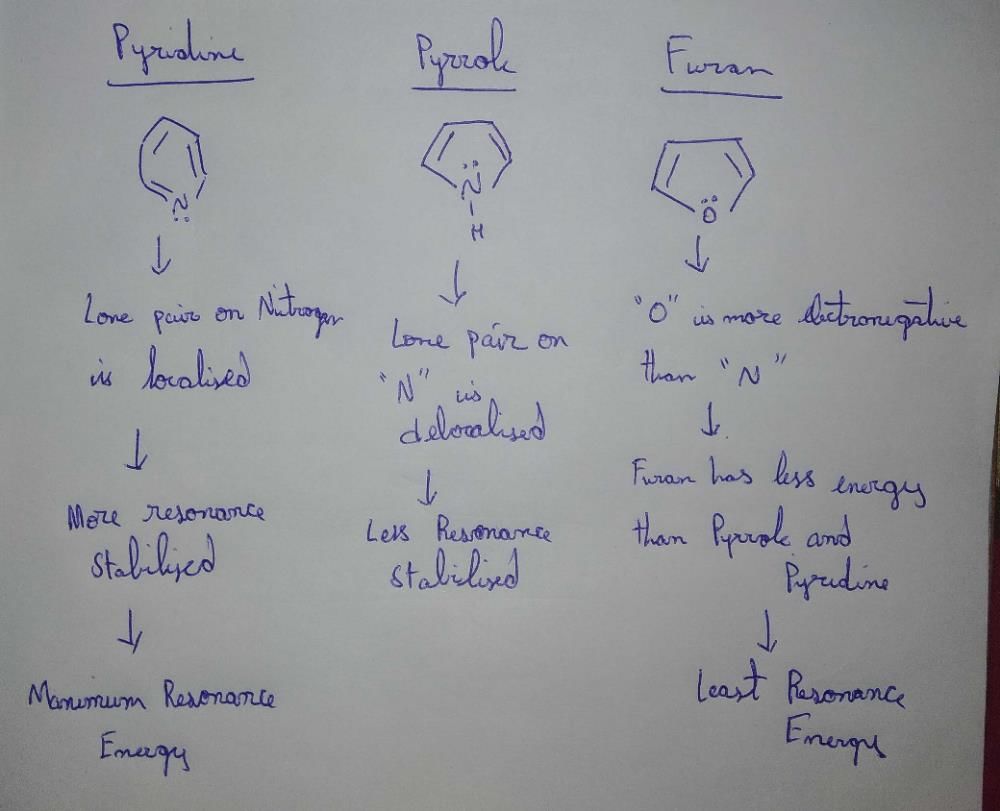
Community Answer
which of the following have highest resonance energy? ...
**Resonance Energy and Aromaticity**
Resonance energy is a measure of the stability of a molecule due to the delocalization of electrons through resonance. Aromatic compounds, which exhibit a high degree of stability due to the delocalization of pi electrons, have high resonance energy. The higher the resonance energy, the more stable the compound.
**Aromaticity in Heterocyclic Compounds**
Heterocyclic compounds are organic compounds that contain one or more heteroatoms (atoms other than carbon) in their ring structure. When discussing aromaticity in heterocyclic compounds, we focus on the delocalization of pi electrons within the ring.
**Comparison of Pyrrole, Furan, and Pyridine**
Pyrrole, furan, and pyridine are all heterocyclic compounds with different ring structures. Let's compare their aromaticity and resonance energy:
**Pyrrole:**
- Pyrrole contains a five-membered ring with four carbon atoms and one nitrogen atom.
- The carbon atoms in pyrrole are sp2 hybridized and participate in pi bonding with the nitrogen atom.
- The nitrogen atom donates a lone pair of electrons into the pi system, creating a delocalized electron cloud.
- Pyrrole is aromatic and exhibits high resonance energy due to the delocalization of six pi electrons in the ring.
- The presence of the lone pair on nitrogen enhances the aromaticity of pyrrole.
**Furan:**
- Furan contains a five-membered ring with four carbon atoms and one oxygen atom.
- The oxygen atom is more electronegative than carbon, leading to a partial positive charge on the carbon atoms adjacent to oxygen.
- The pi electrons in furan are delocalized over the ring, but the presence of the oxygen atom disrupts the aromaticity.
- Furan is aromatic but less stable than pyrrole due to the presence of the electronegative oxygen atom, which withdraws electron density from the ring.
- The lower resonance energy of furan compared to pyrrole is attributed to the destabilizing effect of the oxygen atom.
**Pyridine:**
- Pyridine contains a six-membered ring with five carbon atoms and one nitrogen atom.
- The carbon atoms in pyridine are sp2 hybridized and participate in pi bonding with the nitrogen atom.
- The nitrogen atom is less electronegative than oxygen and does not disrupt the aromaticity of the ring.
- The pi electrons in pyridine are delocalized over the ring, and the compound is aromatic.
- Pyridine has the highest resonance energy among the three compounds because of the greater number of delocalized electrons in the six-membered ring.
**Conclusion**
In summary, pyridine has the highest resonance energy among pyrrole, furan, and pyridine due to the presence of a six-membered ring with a greater number of delocalized electrons. The aromaticity of pyridine is not disrupted by the nitrogen atom, unlike furan, which contains an electronegative oxygen atom.
Resonance energy is a measure of the stability of a molecule due to the delocalization of electrons through resonance. Aromatic compounds, which exhibit a high degree of stability due to the delocalization of pi electrons, have high resonance energy. The higher the resonance energy, the more stable the compound.
**Aromaticity in Heterocyclic Compounds**
Heterocyclic compounds are organic compounds that contain one or more heteroatoms (atoms other than carbon) in their ring structure. When discussing aromaticity in heterocyclic compounds, we focus on the delocalization of pi electrons within the ring.
**Comparison of Pyrrole, Furan, and Pyridine**
Pyrrole, furan, and pyridine are all heterocyclic compounds with different ring structures. Let's compare their aromaticity and resonance energy:
**Pyrrole:**
- Pyrrole contains a five-membered ring with four carbon atoms and one nitrogen atom.
- The carbon atoms in pyrrole are sp2 hybridized and participate in pi bonding with the nitrogen atom.
- The nitrogen atom donates a lone pair of electrons into the pi system, creating a delocalized electron cloud.
- Pyrrole is aromatic and exhibits high resonance energy due to the delocalization of six pi electrons in the ring.
- The presence of the lone pair on nitrogen enhances the aromaticity of pyrrole.
**Furan:**
- Furan contains a five-membered ring with four carbon atoms and one oxygen atom.
- The oxygen atom is more electronegative than carbon, leading to a partial positive charge on the carbon atoms adjacent to oxygen.
- The pi electrons in furan are delocalized over the ring, but the presence of the oxygen atom disrupts the aromaticity.
- Furan is aromatic but less stable than pyrrole due to the presence of the electronegative oxygen atom, which withdraws electron density from the ring.
- The lower resonance energy of furan compared to pyrrole is attributed to the destabilizing effect of the oxygen atom.
**Pyridine:**
- Pyridine contains a six-membered ring with five carbon atoms and one nitrogen atom.
- The carbon atoms in pyridine are sp2 hybridized and participate in pi bonding with the nitrogen atom.
- The nitrogen atom is less electronegative than oxygen and does not disrupt the aromaticity of the ring.
- The pi electrons in pyridine are delocalized over the ring, and the compound is aromatic.
- Pyridine has the highest resonance energy among the three compounds because of the greater number of delocalized electrons in the six-membered ring.
**Conclusion**
In summary, pyridine has the highest resonance energy among pyrrole, furan, and pyridine due to the presence of a six-membered ring with a greater number of delocalized electrons. The aromaticity of pyridine is not disrupted by the nitrogen atom, unlike furan, which contains an electronegative oxygen atom.
Attention Class 10 Students!
To make sure you are not studying endlessly, EduRev has designed Class 10 study material, with Structured Courses, Videos, & Test Series. Plus get personalized analysis, doubt solving and improvement plans to achieve a great score in Class 10.

|
Explore Courses for Class 10 exam
|

|
Similar Class 10 Doubts
which of the following have highest resonance energy? a)pyrrole b)furan c)pyridine. correct option is c)pyridine. can you explain wgy?(why*?) Related: Doubts. Please write solutions?
Question Description
which of the following have highest resonance energy? a)pyrrole b)furan c)pyridine. correct option is c)pyridine. can you explain wgy?(why*?) Related: Doubts. Please write solutions? for Class 10 2024 is part of Class 10 preparation. The Question and answers have been prepared according to the Class 10 exam syllabus. Information about which of the following have highest resonance energy? a)pyrrole b)furan c)pyridine. correct option is c)pyridine. can you explain wgy?(why*?) Related: Doubts. Please write solutions? covers all topics & solutions for Class 10 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for which of the following have highest resonance energy? a)pyrrole b)furan c)pyridine. correct option is c)pyridine. can you explain wgy?(why*?) Related: Doubts. Please write solutions?.
which of the following have highest resonance energy? a)pyrrole b)furan c)pyridine. correct option is c)pyridine. can you explain wgy?(why*?) Related: Doubts. Please write solutions? for Class 10 2024 is part of Class 10 preparation. The Question and answers have been prepared according to the Class 10 exam syllabus. Information about which of the following have highest resonance energy? a)pyrrole b)furan c)pyridine. correct option is c)pyridine. can you explain wgy?(why*?) Related: Doubts. Please write solutions? covers all topics & solutions for Class 10 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for which of the following have highest resonance energy? a)pyrrole b)furan c)pyridine. correct option is c)pyridine. can you explain wgy?(why*?) Related: Doubts. Please write solutions?.
Solutions for which of the following have highest resonance energy? a)pyrrole b)furan c)pyridine. correct option is c)pyridine. can you explain wgy?(why*?) Related: Doubts. Please write solutions? in English & in Hindi are available as part of our courses for Class 10.
Download more important topics, notes, lectures and mock test series for Class 10 Exam by signing up for free.
Here you can find the meaning of which of the following have highest resonance energy? a)pyrrole b)furan c)pyridine. correct option is c)pyridine. can you explain wgy?(why*?) Related: Doubts. Please write solutions? defined & explained in the simplest way possible. Besides giving the explanation of
which of the following have highest resonance energy? a)pyrrole b)furan c)pyridine. correct option is c)pyridine. can you explain wgy?(why*?) Related: Doubts. Please write solutions?, a detailed solution for which of the following have highest resonance energy? a)pyrrole b)furan c)pyridine. correct option is c)pyridine. can you explain wgy?(why*?) Related: Doubts. Please write solutions? has been provided alongside types of which of the following have highest resonance energy? a)pyrrole b)furan c)pyridine. correct option is c)pyridine. can you explain wgy?(why*?) Related: Doubts. Please write solutions? theory, EduRev gives you an
ample number of questions to practice which of the following have highest resonance energy? a)pyrrole b)furan c)pyridine. correct option is c)pyridine. can you explain wgy?(why*?) Related: Doubts. Please write solutions? tests, examples and also practice Class 10 tests.

|
Explore Courses for Class 10 exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.

























