UPSC Exam > UPSC Questions > In graphite carbon atoms are arranged in laye...
Start Learning for Free
In graphite carbon atoms are arranged in layers of
- a)pentagonal arrays
- b)heptagonal arrays
- c)octagonal arrays
- d)hexagonal arrays
Correct answer is option 'D'. Can you explain this answer?
Verified Answer
In graphite carbon atoms are arranged in layers ofa)pentagonal arraysb...
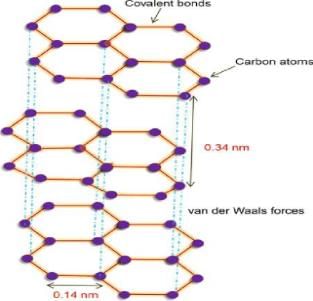
Graphite is composed of layers of carbon atoms that are arranged in 6-membered, hexagonal rings. In this bonding mode the bond angle between adjacent carbon atoms is 120. These “ring arrays” are arranged in large sheets of carbon atoms, and individual sheets are known as graphene layers.

Most Upvoted Answer
In graphite carbon atoms are arranged in layers ofa)pentagonal arraysb...
Graphite Structure
Graphite is a form of carbon in which carbon atoms are arranged in a layered structure. Each layer consists of a hexagonal array of carbon atoms that are bonded together in a unique way. These layers are stacked on top of each other, forming a crystal lattice structure.
Hexagonal Arrays
In graphite, carbon atoms are arranged in hexagonal arrays within each layer. This means that each carbon atom is bonded to three neighboring carbon atoms in the same layer, forming a hexagonal pattern. The carbon-carbon bonds in graphite are strong and covalent in nature.
Layered Structure
The hexagonal arrays of carbon atoms in graphite form individual layers. These layers are separated by weak van der Waals forces, which allow the layers to slide over each other. This sliding property is what gives graphite its characteristic soft and slippery texture.
Properties and Applications
Due to its unique structure, graphite has several important properties. It is an excellent conductor of electricity and heat, making it useful in applications such as electrodes, batteries, and thermal insulators. Graphite is also chemically inert and has a high melting point, making it suitable for high-temperature applications.
Conclusion
In conclusion, carbon atoms in graphite are arranged in layers with hexagonal arrays. Each layer consists of a hexagonal pattern of carbon atoms, with each carbon atom bonded to three neighboring carbon atoms. The layered structure of graphite gives it its unique properties and makes it useful in various applications.
Graphite is a form of carbon in which carbon atoms are arranged in a layered structure. Each layer consists of a hexagonal array of carbon atoms that are bonded together in a unique way. These layers are stacked on top of each other, forming a crystal lattice structure.
Hexagonal Arrays
In graphite, carbon atoms are arranged in hexagonal arrays within each layer. This means that each carbon atom is bonded to three neighboring carbon atoms in the same layer, forming a hexagonal pattern. The carbon-carbon bonds in graphite are strong and covalent in nature.
Layered Structure
The hexagonal arrays of carbon atoms in graphite form individual layers. These layers are separated by weak van der Waals forces, which allow the layers to slide over each other. This sliding property is what gives graphite its characteristic soft and slippery texture.
Properties and Applications
Due to its unique structure, graphite has several important properties. It is an excellent conductor of electricity and heat, making it useful in applications such as electrodes, batteries, and thermal insulators. Graphite is also chemically inert and has a high melting point, making it suitable for high-temperature applications.
Conclusion
In conclusion, carbon atoms in graphite are arranged in layers with hexagonal arrays. Each layer consists of a hexagonal pattern of carbon atoms, with each carbon atom bonded to three neighboring carbon atoms. The layered structure of graphite gives it its unique properties and makes it useful in various applications.

|
Explore Courses for UPSC exam
|

|
Question Description
In graphite carbon atoms are arranged in layers ofa)pentagonal arraysb)heptagonal arraysc)octagonal arraysd)hexagonal arraysCorrect answer is option 'D'. Can you explain this answer? for UPSC 2025 is part of UPSC preparation. The Question and answers have been prepared according to the UPSC exam syllabus. Information about In graphite carbon atoms are arranged in layers ofa)pentagonal arraysb)heptagonal arraysc)octagonal arraysd)hexagonal arraysCorrect answer is option 'D'. Can you explain this answer? covers all topics & solutions for UPSC 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for In graphite carbon atoms are arranged in layers ofa)pentagonal arraysb)heptagonal arraysc)octagonal arraysd)hexagonal arraysCorrect answer is option 'D'. Can you explain this answer?.
In graphite carbon atoms are arranged in layers ofa)pentagonal arraysb)heptagonal arraysc)octagonal arraysd)hexagonal arraysCorrect answer is option 'D'. Can you explain this answer? for UPSC 2025 is part of UPSC preparation. The Question and answers have been prepared according to the UPSC exam syllabus. Information about In graphite carbon atoms are arranged in layers ofa)pentagonal arraysb)heptagonal arraysc)octagonal arraysd)hexagonal arraysCorrect answer is option 'D'. Can you explain this answer? covers all topics & solutions for UPSC 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for In graphite carbon atoms are arranged in layers ofa)pentagonal arraysb)heptagonal arraysc)octagonal arraysd)hexagonal arraysCorrect answer is option 'D'. Can you explain this answer?.
Solutions for In graphite carbon atoms are arranged in layers ofa)pentagonal arraysb)heptagonal arraysc)octagonal arraysd)hexagonal arraysCorrect answer is option 'D'. Can you explain this answer? in English & in Hindi are available as part of our courses for UPSC.
Download more important topics, notes, lectures and mock test series for UPSC Exam by signing up for free.
Here you can find the meaning of In graphite carbon atoms are arranged in layers ofa)pentagonal arraysb)heptagonal arraysc)octagonal arraysd)hexagonal arraysCorrect answer is option 'D'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
In graphite carbon atoms are arranged in layers ofa)pentagonal arraysb)heptagonal arraysc)octagonal arraysd)hexagonal arraysCorrect answer is option 'D'. Can you explain this answer?, a detailed solution for In graphite carbon atoms are arranged in layers ofa)pentagonal arraysb)heptagonal arraysc)octagonal arraysd)hexagonal arraysCorrect answer is option 'D'. Can you explain this answer? has been provided alongside types of In graphite carbon atoms are arranged in layers ofa)pentagonal arraysb)heptagonal arraysc)octagonal arraysd)hexagonal arraysCorrect answer is option 'D'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice In graphite carbon atoms are arranged in layers ofa)pentagonal arraysb)heptagonal arraysc)octagonal arraysd)hexagonal arraysCorrect answer is option 'D'. Can you explain this answer? tests, examples and also practice UPSC tests.

|
Explore Courses for UPSC exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















