NEET Exam > NEET Questions > Trick to remember "Acidic;Basic;Neutral amino...
Start Learning for Free
Trick to remember "Acidic;Basic;Neutral aminoacids" respectively?
Most Upvoted Answer
Trick to remember "Acidic;Basic;Neutral aminoacids" respectively?
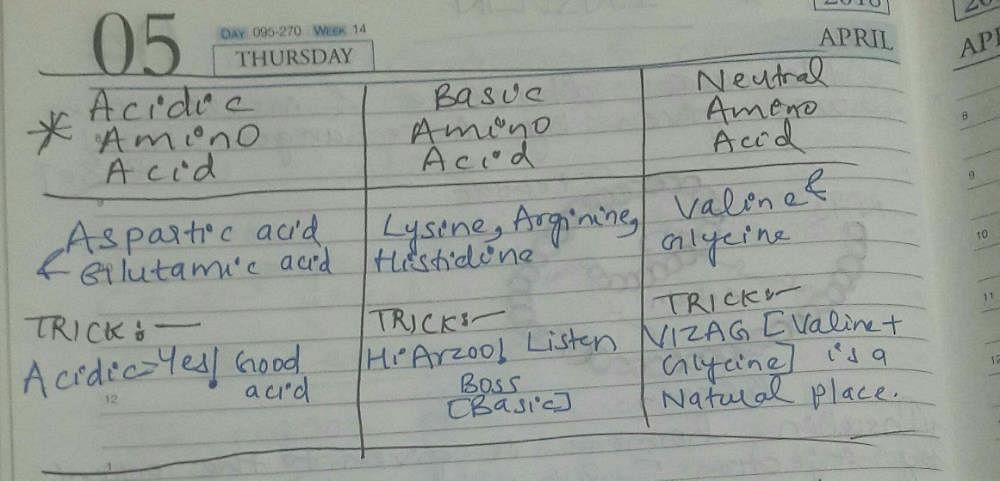
Community Answer
Trick to remember "Acidic;Basic;Neutral aminoacids" respectively?
Acidic, Basic, and Neutral Amino Acids
Amino acids are the building blocks of proteins and are classified into different categories based on their chemical properties. One way to categorize amino acids is by their acidity or basicity, which refers to their ability to release or accept protons (H+ ions) in a solution. There are three main types of amino acids based on their acidity or basicity: acidic amino acids, basic amino acids, and neutral amino acids.
1. Acidic Amino Acids:
Acidic amino acids have a negatively charged side chain at physiological pH, which means they can release protons. The two common acidic amino acids are aspartic acid (Asp) and glutamic acid (Glu). These amino acids play important roles in various biological processes, including enzyme activity and protein-protein interactions.
- Aspartic acid (Asp): Aspartic acid has a side chain that contains a carboxylic acid group (-COOH). It is negatively charged at physiological pH and is therefore considered acidic.
- Glutamic acid (Glu): Glutamic acid also has a side chain with a carboxylic acid group (-COOH) and is negatively charged at physiological pH.
2. Basic Amino Acids:
Basic amino acids have a positively charged side chain at physiological pH, allowing them to accept protons. There are three common basic amino acids: lysine (Lys), arginine (Arg), and histidine (His). These amino acids are involved in various biological functions, including DNA binding, enzyme catalysis, and protein stability.
- Lysine (Lys): Lysine has a side chain that contains an amino group (-NH2), making it positively charged at physiological pH.
- Arginine (Arg): Arginine also has a side chain with an amino group (-NH2) but contains an additional guanidinium group (-NH-C(NH2)2), which contributes to its positive charge.
- Histidine (His): Histidine has a side chain with an imidazole group, which can be either positively charged or neutral depending on the pH of the environment.
3. Neutral Amino Acids:
Neutral amino acids have side chains that do not possess a significant charge at physiological pH. They can be further classified into polar and nonpolar amino acids based on the nature of their side chains.
- Polar neutral amino acids: Polar neutral amino acids have side chains that contain functional groups such as hydroxyl (-OH), thiol (-SH), or amide (-CONH2) groups. Examples of polar neutral amino acids include serine (Ser), threonine (Thr), and asparagine (Asn).
- Nonpolar neutral amino acids: Nonpolar neutral amino acids have side chains that are composed of hydrogen and carbon atoms only. They tend to be hydrophobic and are often found buried within the interior of proteins. Examples of nonpolar neutral amino acids include glycine (Gly), alanine (Ala), and valine (Val).
Remembering the classification of acidic, basic, and neutral amino acids can be made easier by understanding their chemical properties and structures. Mnemonic devices, flashcards, or practice quizzes can also help reinforce the information and aid in memorization.
Amino acids are the building blocks of proteins and are classified into different categories based on their chemical properties. One way to categorize amino acids is by their acidity or basicity, which refers to their ability to release or accept protons (H+ ions) in a solution. There are three main types of amino acids based on their acidity or basicity: acidic amino acids, basic amino acids, and neutral amino acids.
1. Acidic Amino Acids:
Acidic amino acids have a negatively charged side chain at physiological pH, which means they can release protons. The two common acidic amino acids are aspartic acid (Asp) and glutamic acid (Glu). These amino acids play important roles in various biological processes, including enzyme activity and protein-protein interactions.
- Aspartic acid (Asp): Aspartic acid has a side chain that contains a carboxylic acid group (-COOH). It is negatively charged at physiological pH and is therefore considered acidic.
- Glutamic acid (Glu): Glutamic acid also has a side chain with a carboxylic acid group (-COOH) and is negatively charged at physiological pH.
2. Basic Amino Acids:
Basic amino acids have a positively charged side chain at physiological pH, allowing them to accept protons. There are three common basic amino acids: lysine (Lys), arginine (Arg), and histidine (His). These amino acids are involved in various biological functions, including DNA binding, enzyme catalysis, and protein stability.
- Lysine (Lys): Lysine has a side chain that contains an amino group (-NH2), making it positively charged at physiological pH.
- Arginine (Arg): Arginine also has a side chain with an amino group (-NH2) but contains an additional guanidinium group (-NH-C(NH2)2), which contributes to its positive charge.
- Histidine (His): Histidine has a side chain with an imidazole group, which can be either positively charged or neutral depending on the pH of the environment.
3. Neutral Amino Acids:
Neutral amino acids have side chains that do not possess a significant charge at physiological pH. They can be further classified into polar and nonpolar amino acids based on the nature of their side chains.
- Polar neutral amino acids: Polar neutral amino acids have side chains that contain functional groups such as hydroxyl (-OH), thiol (-SH), or amide (-CONH2) groups. Examples of polar neutral amino acids include serine (Ser), threonine (Thr), and asparagine (Asn).
- Nonpolar neutral amino acids: Nonpolar neutral amino acids have side chains that are composed of hydrogen and carbon atoms only. They tend to be hydrophobic and are often found buried within the interior of proteins. Examples of nonpolar neutral amino acids include glycine (Gly), alanine (Ala), and valine (Val).
Remembering the classification of acidic, basic, and neutral amino acids can be made easier by understanding their chemical properties and structures. Mnemonic devices, flashcards, or practice quizzes can also help reinforce the information and aid in memorization.
Attention NEET Students!
To make sure you are not studying endlessly, EduRev has designed NEET study material, with Structured Courses, Videos, & Test Series. Plus get personalized analysis, doubt solving and improvement plans to achieve a great score in NEET.

|
Explore Courses for NEET exam
|

|
Similar NEET Doubts
Trick to remember "Acidic;Basic;Neutral aminoacids" respectively?
Question Description
Trick to remember "Acidic;Basic;Neutral aminoacids" respectively? for NEET 2024 is part of NEET preparation. The Question and answers have been prepared according to the NEET exam syllabus. Information about Trick to remember "Acidic;Basic;Neutral aminoacids" respectively? covers all topics & solutions for NEET 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Trick to remember "Acidic;Basic;Neutral aminoacids" respectively?.
Trick to remember "Acidic;Basic;Neutral aminoacids" respectively? for NEET 2024 is part of NEET preparation. The Question and answers have been prepared according to the NEET exam syllabus. Information about Trick to remember "Acidic;Basic;Neutral aminoacids" respectively? covers all topics & solutions for NEET 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Trick to remember "Acidic;Basic;Neutral aminoacids" respectively?.
Solutions for Trick to remember "Acidic;Basic;Neutral aminoacids" respectively? in English & in Hindi are available as part of our courses for NEET.
Download more important topics, notes, lectures and mock test series for NEET Exam by signing up for free.
Here you can find the meaning of Trick to remember "Acidic;Basic;Neutral aminoacids" respectively? defined & explained in the simplest way possible. Besides giving the explanation of
Trick to remember "Acidic;Basic;Neutral aminoacids" respectively?, a detailed solution for Trick to remember "Acidic;Basic;Neutral aminoacids" respectively? has been provided alongside types of Trick to remember "Acidic;Basic;Neutral aminoacids" respectively? theory, EduRev gives you an
ample number of questions to practice Trick to remember "Acidic;Basic;Neutral aminoacids" respectively? tests, examples and also practice NEET tests.

|
Explore Courses for NEET exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.

























