UPSC Exam > UPSC Questions > Which among the following converts chemical ...
Start Learning for Free
Which among the following converts chemical energy to electrical energy?
- a)Motor
- b)Dynamo
- c)Battery
- d)Generator
Correct answer is option 'C'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
Which among the following converts chemical energy to electrical ener...
The correct answer is Battery. 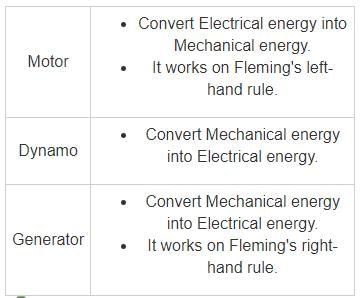
View all questions of this test
- A battery is a device that stores chemical energy and converts it to electrical energy.
- The chemical reactions in a battery involve the flow of electrons from one material (electrode) to another, through an external circuit.
- Energy using an electrochemical oxidation-reduction (redox) reaction.
- The flow of electrons provides an electric current that can be used to do work.
Important Points

Additional Information
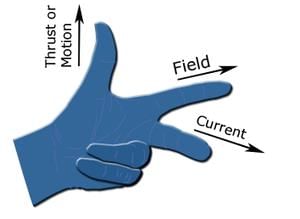
Fleming's right-hand rule (generators):
- It shows the direction of induced current when a conductor attached to a circuit moves in a magnetic field. It can be used to determine the direction of current in a generator's windings.
- The thumb is pointed in the direction of the motion of the conductor relative to the magnetic field.
- The first finger is pointed in the direction of the magnetic field. (North to south).
- Then the second finger represents the direction of the induced or generated current within the conductor (from the terminal with lower electric potential to the terminal with higher electric potential, as in a voltage source).

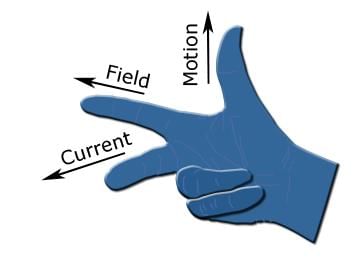
Fleming's left-hand rule (electric motors):
- When current flows through a conducting wire, and an external magnetic field is applied across that flow, the conducting wire experiences a force perpendicular both to that field and to the direction of the current flow (i.e. they are mutually perpendicular).
- The Thumb represents the direction of the Thrust on the conductor / Motion of the Conductor.
- The Forefinger represents the direction of the Magnetic Field.
- The Centre finger represents the direction of the Current.

Key-Points
- The Anode is the negative or reducing electrode that releases electrons to the external circuit and oxidizes during an electrochemical reaction.
- The Cathode is the positive or oxidizing electrode that acquires electrons from the external circuit and is reduced during the electrochemical reaction.
- The Electrolyte is the medium that provides the ion transport mechanism between the cathode and anode of a cell.
- Electrolytes are often thought of as liquids, such as water or other solvents, with dissolved salts, acids, or alkalis that are required for ionic conduction.

|
Explore Courses for UPSC exam
|

|
Similar UPSC Doubts
Which among the following converts chemical energy to electrical energy?a)Motorb)Dynamoc)Batteryd)GeneratorCorrect answer is option 'C'. Can you explain this answer?
Question Description
Which among the following converts chemical energy to electrical energy?a)Motorb)Dynamoc)Batteryd)GeneratorCorrect answer is option 'C'. Can you explain this answer? for UPSC 2024 is part of UPSC preparation. The Question and answers have been prepared according to the UPSC exam syllabus. Information about Which among the following converts chemical energy to electrical energy?a)Motorb)Dynamoc)Batteryd)GeneratorCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for UPSC 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Which among the following converts chemical energy to electrical energy?a)Motorb)Dynamoc)Batteryd)GeneratorCorrect answer is option 'C'. Can you explain this answer?.
Which among the following converts chemical energy to electrical energy?a)Motorb)Dynamoc)Batteryd)GeneratorCorrect answer is option 'C'. Can you explain this answer? for UPSC 2024 is part of UPSC preparation. The Question and answers have been prepared according to the UPSC exam syllabus. Information about Which among the following converts chemical energy to electrical energy?a)Motorb)Dynamoc)Batteryd)GeneratorCorrect answer is option 'C'. Can you explain this answer? covers all topics & solutions for UPSC 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Which among the following converts chemical energy to electrical energy?a)Motorb)Dynamoc)Batteryd)GeneratorCorrect answer is option 'C'. Can you explain this answer?.
Solutions for Which among the following converts chemical energy to electrical energy?a)Motorb)Dynamoc)Batteryd)GeneratorCorrect answer is option 'C'. Can you explain this answer? in English & in Hindi are available as part of our courses for UPSC.
Download more important topics, notes, lectures and mock test series for UPSC Exam by signing up for free.
Here you can find the meaning of Which among the following converts chemical energy to electrical energy?a)Motorb)Dynamoc)Batteryd)GeneratorCorrect answer is option 'C'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Which among the following converts chemical energy to electrical energy?a)Motorb)Dynamoc)Batteryd)GeneratorCorrect answer is option 'C'. Can you explain this answer?, a detailed solution for Which among the following converts chemical energy to electrical energy?a)Motorb)Dynamoc)Batteryd)GeneratorCorrect answer is option 'C'. Can you explain this answer? has been provided alongside types of Which among the following converts chemical energy to electrical energy?a)Motorb)Dynamoc)Batteryd)GeneratorCorrect answer is option 'C'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Which among the following converts chemical energy to electrical energy?a)Motorb)Dynamoc)Batteryd)GeneratorCorrect answer is option 'C'. Can you explain this answer? tests, examples and also practice UPSC tests.

|
Explore Courses for UPSC exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.























