NEET Exam > NEET Questions > Which of the following ions is the most reson...
Start Learning for Free
Which of the following ions is the most resonance stabilised?
- a)Ethoxide
- b)Phenoxide
- c)Butoxide
- d)Isopropoxide
Correct answer is option 'B'. Can you explain this answer?
Most Upvoted Answer
Which of the following ions is the most resonance stabilised?a)Ethoxid...
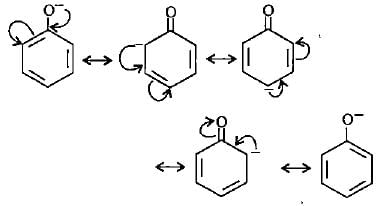
Phenoxide ion shows maximum resonating structures.

Free Test
FREE
| Start Free Test |
Community Answer
Which of the following ions is the most resonance stabilised?a)Ethoxid...
Resonance Stabilization of Phenoxide Ion
Phenoxide ion (C6H5O-) is the most resonance stabilized among the given ions. Resonance stabilization occurs when electron delocalization is possible through the interaction of p-orbitals. Let's explore the reasons why phenoxide ion is the most resonance stabilized.
Structure of Phenoxide Ion
The phenoxide ion is derived from phenol (C6H5OH) by removing a proton from the hydroxyl group. The resulting ion has a negative charge on the oxygen atom and a lone pair of electrons.
Resonance Structures
Phenoxide ion can be represented by multiple resonance structures due to the delocalization of electrons. Resonance structures are different representations of a molecule or ion that differ only in the placement of electrons.
Resonance Forms of Phenoxide Ion
1. In the first resonance structure, the negative charge is on the oxygen atom, and the lone pair of electrons is on the carbon atom adjacent to the oxygen. This structure is stabilized by the electronegative oxygen atom.
2. In the second resonance structure, the negative charge is on the carbon atom adjacent to the oxygen, and the lone pair of electrons is on the oxygen atom. This structure is stabilized by the delocalization of the negative charge.
3. In the third resonance structure, the negative charge is delocalized over the entire benzene ring, and the lone pair of electrons is on the oxygen atom. This structure is further stabilized by the aromaticity of the benzene ring.
Stability of Phenoxide Ion
The delocalization of the negative charge and the lone pair of electrons in phenoxide ion results in greater stability compared to the other ions. The stability is attributed to the following factors:
1. Aromaticity: The benzene ring in phenoxide ion is aromatic, which provides additional stability to the ion.
2. Delocalization of charge: The negative charge is delocalized over the oxygen atom, adjacent carbon atom, and the benzene ring. This delocalization spreads the charge and stabilizes the ion.
3. Electron-withdrawing effect: The electronegative oxygen atom withdraws electron density from the benzene ring, causing it to become more stable.
4. Conjugation: The conjugation between the oxygen atom, adjacent carbon atom, and the benzene ring allows for the delocalization of electrons, resulting in greater stability.
Overall, the combination of aromaticity, charge delocalization, electron-withdrawing effect, and conjugation makes phenoxide ion the most resonance stabilized among the given ions.
Phenoxide ion (C6H5O-) is the most resonance stabilized among the given ions. Resonance stabilization occurs when electron delocalization is possible through the interaction of p-orbitals. Let's explore the reasons why phenoxide ion is the most resonance stabilized.
Structure of Phenoxide Ion
The phenoxide ion is derived from phenol (C6H5OH) by removing a proton from the hydroxyl group. The resulting ion has a negative charge on the oxygen atom and a lone pair of electrons.
Resonance Structures
Phenoxide ion can be represented by multiple resonance structures due to the delocalization of electrons. Resonance structures are different representations of a molecule or ion that differ only in the placement of electrons.
Resonance Forms of Phenoxide Ion
1. In the first resonance structure, the negative charge is on the oxygen atom, and the lone pair of electrons is on the carbon atom adjacent to the oxygen. This structure is stabilized by the electronegative oxygen atom.
2. In the second resonance structure, the negative charge is on the carbon atom adjacent to the oxygen, and the lone pair of electrons is on the oxygen atom. This structure is stabilized by the delocalization of the negative charge.
3. In the third resonance structure, the negative charge is delocalized over the entire benzene ring, and the lone pair of electrons is on the oxygen atom. This structure is further stabilized by the aromaticity of the benzene ring.
Stability of Phenoxide Ion
The delocalization of the negative charge and the lone pair of electrons in phenoxide ion results in greater stability compared to the other ions. The stability is attributed to the following factors:
1. Aromaticity: The benzene ring in phenoxide ion is aromatic, which provides additional stability to the ion.
2. Delocalization of charge: The negative charge is delocalized over the oxygen atom, adjacent carbon atom, and the benzene ring. This delocalization spreads the charge and stabilizes the ion.
3. Electron-withdrawing effect: The electronegative oxygen atom withdraws electron density from the benzene ring, causing it to become more stable.
4. Conjugation: The conjugation between the oxygen atom, adjacent carbon atom, and the benzene ring allows for the delocalization of electrons, resulting in greater stability.
Overall, the combination of aromaticity, charge delocalization, electron-withdrawing effect, and conjugation makes phenoxide ion the most resonance stabilized among the given ions.

|
Explore Courses for NEET exam
|

|
Question Description
Which of the following ions is the most resonance stabilised?a)Ethoxideb)Phenoxidec)Butoxided)IsopropoxideCorrect answer is option 'B'. Can you explain this answer? for NEET 2025 is part of NEET preparation. The Question and answers have been prepared according to the NEET exam syllabus. Information about Which of the following ions is the most resonance stabilised?a)Ethoxideb)Phenoxidec)Butoxided)IsopropoxideCorrect answer is option 'B'. Can you explain this answer? covers all topics & solutions for NEET 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Which of the following ions is the most resonance stabilised?a)Ethoxideb)Phenoxidec)Butoxided)IsopropoxideCorrect answer is option 'B'. Can you explain this answer?.
Which of the following ions is the most resonance stabilised?a)Ethoxideb)Phenoxidec)Butoxided)IsopropoxideCorrect answer is option 'B'. Can you explain this answer? for NEET 2025 is part of NEET preparation. The Question and answers have been prepared according to the NEET exam syllabus. Information about Which of the following ions is the most resonance stabilised?a)Ethoxideb)Phenoxidec)Butoxided)IsopropoxideCorrect answer is option 'B'. Can you explain this answer? covers all topics & solutions for NEET 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Which of the following ions is the most resonance stabilised?a)Ethoxideb)Phenoxidec)Butoxided)IsopropoxideCorrect answer is option 'B'. Can you explain this answer?.
Solutions for Which of the following ions is the most resonance stabilised?a)Ethoxideb)Phenoxidec)Butoxided)IsopropoxideCorrect answer is option 'B'. Can you explain this answer? in English & in Hindi are available as part of our courses for NEET.
Download more important topics, notes, lectures and mock test series for NEET Exam by signing up for free.
Here you can find the meaning of Which of the following ions is the most resonance stabilised?a)Ethoxideb)Phenoxidec)Butoxided)IsopropoxideCorrect answer is option 'B'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Which of the following ions is the most resonance stabilised?a)Ethoxideb)Phenoxidec)Butoxided)IsopropoxideCorrect answer is option 'B'. Can you explain this answer?, a detailed solution for Which of the following ions is the most resonance stabilised?a)Ethoxideb)Phenoxidec)Butoxided)IsopropoxideCorrect answer is option 'B'. Can you explain this answer? has been provided alongside types of Which of the following ions is the most resonance stabilised?a)Ethoxideb)Phenoxidec)Butoxided)IsopropoxideCorrect answer is option 'B'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Which of the following ions is the most resonance stabilised?a)Ethoxideb)Phenoxidec)Butoxided)IsopropoxideCorrect answer is option 'B'. Can you explain this answer? tests, examples and also practice NEET tests.

|
Explore Courses for NEET exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















