NEET Exam > NEET Questions > Though covalent in nature, methanol is solubl...
Start Learning for Free
Though covalent in nature, methanol is soluble in water, why?
- a)Methanol is transparent like water
- b)Due to hydrogen bonding between methanol and water molecules
- c)Due to van der Waals' forces between methanoland water
- d)Due to covalent attraction forces
Correct answer is option 'B'. Can you explain this answer?
Most Upvoted Answer
Though covalent in nature, methanol is soluble in water, why?a)Methano...
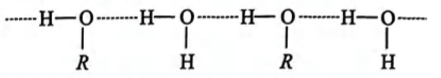
In methanol, R is —CH3 group.
Hydrogen bonding between methanol and water.
Free Test
FREE
| Start Free Test |
Community Answer
Though covalent in nature, methanol is soluble in water, why?a)Methano...
Explanation:
Methanol is a covalent compound with the chemical formula CH3OH. It consists of a carbon atom bonded to three hydrogen atoms and one oxygen atom. The oxygen atom is also bonded to the carbon atom through a covalent bond.
Methanol is soluble in water due to the presence of hydrogen bonding between methanol and water molecules.
Hydrogen Bonding:
Hydrogen bonding occurs when a hydrogen atom is bonded to an electronegative atom (such as oxygen or nitrogen) and is attracted to another electronegative atom nearby. In water molecules, the oxygen atom is more electronegative than the hydrogen atoms, giving rise to a partial negative charge on the oxygen atom and a partial positive charge on the hydrogen atoms.
In methanol, the oxygen atom is also electronegative, and the hydrogen atom bonded to it is partially positive. This allows the oxygen atom in methanol to form hydrogen bonds with the oxygen and hydrogen atoms in water molecules. The partial positive charge on the hydrogen atoms in water molecules can interact with the partial negative charge on the oxygen atom in methanol, and vice versa, forming hydrogen bonds.
Benefits of hydrogen bonding:
The presence of hydrogen bonding between methanol and water molecules allows them to interact and mix together. This leads to the dissolution of methanol in water.
Hydrogen bonding is a relatively strong intermolecular force, so it overcomes the weaker van der Waals forces between methanol molecules. Van der Waals forces are attractive forces between molecules, but they are weaker than hydrogen bonding. Therefore, the hydrogen bonding between methanol and water molecules is the primary reason for the solubility of methanol in water.
Conclusion:
In conclusion, methanol is soluble in water due to the presence of hydrogen bonding between methanol and water molecules. This allows them to mix together and form a homogeneous solution.
Methanol is a covalent compound with the chemical formula CH3OH. It consists of a carbon atom bonded to three hydrogen atoms and one oxygen atom. The oxygen atom is also bonded to the carbon atom through a covalent bond.
Methanol is soluble in water due to the presence of hydrogen bonding between methanol and water molecules.
Hydrogen Bonding:
Hydrogen bonding occurs when a hydrogen atom is bonded to an electronegative atom (such as oxygen or nitrogen) and is attracted to another electronegative atom nearby. In water molecules, the oxygen atom is more electronegative than the hydrogen atoms, giving rise to a partial negative charge on the oxygen atom and a partial positive charge on the hydrogen atoms.
In methanol, the oxygen atom is also electronegative, and the hydrogen atom bonded to it is partially positive. This allows the oxygen atom in methanol to form hydrogen bonds with the oxygen and hydrogen atoms in water molecules. The partial positive charge on the hydrogen atoms in water molecules can interact with the partial negative charge on the oxygen atom in methanol, and vice versa, forming hydrogen bonds.
Benefits of hydrogen bonding:
The presence of hydrogen bonding between methanol and water molecules allows them to interact and mix together. This leads to the dissolution of methanol in water.
Hydrogen bonding is a relatively strong intermolecular force, so it overcomes the weaker van der Waals forces between methanol molecules. Van der Waals forces are attractive forces between molecules, but they are weaker than hydrogen bonding. Therefore, the hydrogen bonding between methanol and water molecules is the primary reason for the solubility of methanol in water.
Conclusion:
In conclusion, methanol is soluble in water due to the presence of hydrogen bonding between methanol and water molecules. This allows them to mix together and form a homogeneous solution.

|
Explore Courses for NEET exam
|

|
Question Description
Though covalent in nature, methanol is soluble in water, why?a)Methanol is transparent like waterb)Due to hydrogen bonding between methanol and water moleculesc)Due to van der Waals forces between methanoland waterd)Due to covalent attraction forcesCorrect answer is option 'B'. Can you explain this answer? for NEET 2025 is part of NEET preparation. The Question and answers have been prepared according to the NEET exam syllabus. Information about Though covalent in nature, methanol is soluble in water, why?a)Methanol is transparent like waterb)Due to hydrogen bonding between methanol and water moleculesc)Due to van der Waals forces between methanoland waterd)Due to covalent attraction forcesCorrect answer is option 'B'. Can you explain this answer? covers all topics & solutions for NEET 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Though covalent in nature, methanol is soluble in water, why?a)Methanol is transparent like waterb)Due to hydrogen bonding between methanol and water moleculesc)Due to van der Waals forces between methanoland waterd)Due to covalent attraction forcesCorrect answer is option 'B'. Can you explain this answer?.
Though covalent in nature, methanol is soluble in water, why?a)Methanol is transparent like waterb)Due to hydrogen bonding between methanol and water moleculesc)Due to van der Waals forces between methanoland waterd)Due to covalent attraction forcesCorrect answer is option 'B'. Can you explain this answer? for NEET 2025 is part of NEET preparation. The Question and answers have been prepared according to the NEET exam syllabus. Information about Though covalent in nature, methanol is soluble in water, why?a)Methanol is transparent like waterb)Due to hydrogen bonding between methanol and water moleculesc)Due to van der Waals forces between methanoland waterd)Due to covalent attraction forcesCorrect answer is option 'B'. Can you explain this answer? covers all topics & solutions for NEET 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Though covalent in nature, methanol is soluble in water, why?a)Methanol is transparent like waterb)Due to hydrogen bonding between methanol and water moleculesc)Due to van der Waals forces between methanoland waterd)Due to covalent attraction forcesCorrect answer is option 'B'. Can you explain this answer?.
Solutions for Though covalent in nature, methanol is soluble in water, why?a)Methanol is transparent like waterb)Due to hydrogen bonding between methanol and water moleculesc)Due to van der Waals forces between methanoland waterd)Due to covalent attraction forcesCorrect answer is option 'B'. Can you explain this answer? in English & in Hindi are available as part of our courses for NEET.
Download more important topics, notes, lectures and mock test series for NEET Exam by signing up for free.
Here you can find the meaning of Though covalent in nature, methanol is soluble in water, why?a)Methanol is transparent like waterb)Due to hydrogen bonding between methanol and water moleculesc)Due to van der Waals forces between methanoland waterd)Due to covalent attraction forcesCorrect answer is option 'B'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Though covalent in nature, methanol is soluble in water, why?a)Methanol is transparent like waterb)Due to hydrogen bonding between methanol and water moleculesc)Due to van der Waals forces between methanoland waterd)Due to covalent attraction forcesCorrect answer is option 'B'. Can you explain this answer?, a detailed solution for Though covalent in nature, methanol is soluble in water, why?a)Methanol is transparent like waterb)Due to hydrogen bonding between methanol and water moleculesc)Due to van der Waals forces between methanoland waterd)Due to covalent attraction forcesCorrect answer is option 'B'. Can you explain this answer? has been provided alongside types of Though covalent in nature, methanol is soluble in water, why?a)Methanol is transparent like waterb)Due to hydrogen bonding between methanol and water moleculesc)Due to van der Waals forces between methanoland waterd)Due to covalent attraction forcesCorrect answer is option 'B'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Though covalent in nature, methanol is soluble in water, why?a)Methanol is transparent like waterb)Due to hydrogen bonding between methanol and water moleculesc)Due to van der Waals forces between methanoland waterd)Due to covalent attraction forcesCorrect answer is option 'B'. Can you explain this answer? tests, examples and also practice NEET tests.

|
Explore Courses for NEET exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.























