NEET Exam > NEET Questions > Two identical glass (μg = 3/2)equiconvex l...
Start Learning for Free
Two identical glass (μg = 3/2) equiconvex lenses of focal length f each are kept in contact. The space between the two lenses is filled with water (μw = 4/3). The focal length of the combination is
- a)f
- b)f/2
- c)4f/3
- d)3f/4
Correct answer is option 'D'. Can you explain this answer?
Verified Answer
Two identical glass (μg = 3/2)equiconvex lenses of focal length f e...
Let R be the radius of curvature of each surface. Then
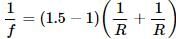
For the water lens
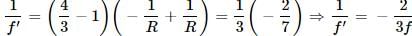
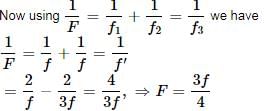
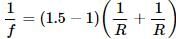
For the water lens
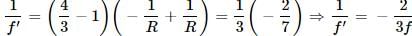
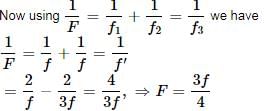
Most Upvoted Answer
Two identical glass (μg = 3/2)equiconvex lenses of focal length f e...
) bottles are filled with water. One bottle is left uncovered, while the other is sealed with a cap. After some time, you observe that the water level in the uncovered bottle has decreased, while the water level in the sealed bottle remains the same.
This phenomenon can be explained by the process of evaporation. When a liquid is left uncovered, its molecules at the surface gain enough energy from the surroundings to escape into the surrounding air as vapors. This process is called evaporation.
In the case of the uncovered bottle, the water molecules on the surface continuously evaporate, causing the water level to decrease over time. However, in the sealed bottle, the cap prevents the water molecules from escaping into the surrounding air, so there is no evaporation occurring. As a result, the water level remains the same in the sealed bottle.
Overall, the difference in water level between the uncovered and sealed bottles is due to the evaporation process, which only occurs in the uncovered bottle.
This phenomenon can be explained by the process of evaporation. When a liquid is left uncovered, its molecules at the surface gain enough energy from the surroundings to escape into the surrounding air as vapors. This process is called evaporation.
In the case of the uncovered bottle, the water molecules on the surface continuously evaporate, causing the water level to decrease over time. However, in the sealed bottle, the cap prevents the water molecules from escaping into the surrounding air, so there is no evaporation occurring. As a result, the water level remains the same in the sealed bottle.
Overall, the difference in water level between the uncovered and sealed bottles is due to the evaporation process, which only occurs in the uncovered bottle.

|
Explore Courses for NEET exam
|

|
Question Description
Two identical glass (μg = 3/2)equiconvex lenses of focal length f each are kept in contact. The space between the two lenses is filled with water (μw = 4/3). The focal length of the combination isa)fb)f/2c)4f/3d)3f/4Correct answer is option 'D'. Can you explain this answer? for NEET 2025 is part of NEET preparation. The Question and answers have been prepared according to the NEET exam syllabus. Information about Two identical glass (μg = 3/2)equiconvex lenses of focal length f each are kept in contact. The space between the two lenses is filled with water (μw = 4/3). The focal length of the combination isa)fb)f/2c)4f/3d)3f/4Correct answer is option 'D'. Can you explain this answer? covers all topics & solutions for NEET 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Two identical glass (μg = 3/2)equiconvex lenses of focal length f each are kept in contact. The space between the two lenses is filled with water (μw = 4/3). The focal length of the combination isa)fb)f/2c)4f/3d)3f/4Correct answer is option 'D'. Can you explain this answer?.
Two identical glass (μg = 3/2)equiconvex lenses of focal length f each are kept in contact. The space between the two lenses is filled with water (μw = 4/3). The focal length of the combination isa)fb)f/2c)4f/3d)3f/4Correct answer is option 'D'. Can you explain this answer? for NEET 2025 is part of NEET preparation. The Question and answers have been prepared according to the NEET exam syllabus. Information about Two identical glass (μg = 3/2)equiconvex lenses of focal length f each are kept in contact. The space between the two lenses is filled with water (μw = 4/3). The focal length of the combination isa)fb)f/2c)4f/3d)3f/4Correct answer is option 'D'. Can you explain this answer? covers all topics & solutions for NEET 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Two identical glass (μg = 3/2)equiconvex lenses of focal length f each are kept in contact. The space between the two lenses is filled with water (μw = 4/3). The focal length of the combination isa)fb)f/2c)4f/3d)3f/4Correct answer is option 'D'. Can you explain this answer?.
Solutions for Two identical glass (μg = 3/2)equiconvex lenses of focal length f each are kept in contact. The space between the two lenses is filled with water (μw = 4/3). The focal length of the combination isa)fb)f/2c)4f/3d)3f/4Correct answer is option 'D'. Can you explain this answer? in English & in Hindi are available as part of our courses for NEET.
Download more important topics, notes, lectures and mock test series for NEET Exam by signing up for free.
Here you can find the meaning of Two identical glass (μg = 3/2)equiconvex lenses of focal length f each are kept in contact. The space between the two lenses is filled with water (μw = 4/3). The focal length of the combination isa)fb)f/2c)4f/3d)3f/4Correct answer is option 'D'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Two identical glass (μg = 3/2)equiconvex lenses of focal length f each are kept in contact. The space between the two lenses is filled with water (μw = 4/3). The focal length of the combination isa)fb)f/2c)4f/3d)3f/4Correct answer is option 'D'. Can you explain this answer?, a detailed solution for Two identical glass (μg = 3/2)equiconvex lenses of focal length f each are kept in contact. The space between the two lenses is filled with water (μw = 4/3). The focal length of the combination isa)fb)f/2c)4f/3d)3f/4Correct answer is option 'D'. Can you explain this answer? has been provided alongside types of Two identical glass (μg = 3/2)equiconvex lenses of focal length f each are kept in contact. The space between the two lenses is filled with water (μw = 4/3). The focal length of the combination isa)fb)f/2c)4f/3d)3f/4Correct answer is option 'D'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Two identical glass (μg = 3/2)equiconvex lenses of focal length f each are kept in contact. The space between the two lenses is filled with water (μw = 4/3). The focal length of the combination isa)fb)f/2c)4f/3d)3f/4Correct answer is option 'D'. Can you explain this answer? tests, examples and also practice NEET tests.

|
Explore Courses for NEET exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















