NEET Exam > NEET Questions > In a ccp structure, if 2/3 rd face-centred a...
Start Learning for Free
In a ccp structure, if 2/3 rd face-centred atoms are removed, then the percentage of occupied space in unit cell will be
- a)60%
- b)2.2%
- c)47.5 %
- d)37%
Correct answer is option 'D'. Can you explain this answer?
Most Upvoted Answer
In a ccp structure, if 2/3 rd face-centred atoms are removed, then th...
To understand why the correct answer is option 'D' (37%), let's break down the explanation into several sections:
1. Understanding the Face-Centered Cubic (FCC) Structure:
The face-centered cubic (FCC) structure is a type of crystal lattice arrangement commonly found in metals. In this structure, the unit cell consists of atoms at the eight corners of the cube and additional atoms at the centers of each face. This arrangement allows for efficient packing of atoms and provides high symmetry.
2. Calculation of Occupied Space:
To calculate the percentage of occupied space in the unit cell, we need to consider the number of atoms present in the unit cell after removing 2/3 of the face-centered atoms.
3. Initial Number of Face-Centered Atoms:
In an FCC structure, each face of the unit cell contains one atom. Since there are six faces, there are a total of 6 face-centered atoms in the unit cell.
4. Number of Face-Centered Atoms Removed:
If 2/3 of the face-centered atoms are removed, we need to calculate 2/3 of 6.
2/3 * 6 = 4
Thus, 4 face-centered atoms are removed from the unit cell.
5. Remaining Face-Centered Atoms:
After removing 4 face-centered atoms, we are left with 6 - 4 = 2 face-centered atoms in the unit cell.
6. Total Number of Atoms in the Unit Cell:
In addition to the face-centered atoms, there are also atoms at the eight corners of the unit cell. Each corner atom is shared between eight neighboring unit cells. Therefore, the number of corner atoms within the unit cell is 1/8.
Thus, the total number of atoms in the unit cell can be calculated as follows:
Number of corner atoms = 8 * 1/8 = 1
Number of face-centered atoms = 2
Total number of atoms = Number of corner atoms + Number of face-centered atoms = 1 + 2 = 3
7. Calculation of Occupied Space:
To calculate the percentage of occupied space, we need to consider the volume occupied by the atoms in the unit cell. In an FCC structure, each atom occupies 1/8th of the unit cell volume.
Percentage of occupied space = (Number of atoms * Volume occupied by each atom) / Total volume of the unit cell * 100%
Percentage of occupied space = (3 * 1/8) / 1 * 100% = 3/8 * 100% = 37%
Therefore, the correct answer is option 'D' (37%), which represents the percentage of occupied space in the unit cell after removing 2/3 of the face-centered atoms.
1. Understanding the Face-Centered Cubic (FCC) Structure:
The face-centered cubic (FCC) structure is a type of crystal lattice arrangement commonly found in metals. In this structure, the unit cell consists of atoms at the eight corners of the cube and additional atoms at the centers of each face. This arrangement allows for efficient packing of atoms and provides high symmetry.
2. Calculation of Occupied Space:
To calculate the percentage of occupied space in the unit cell, we need to consider the number of atoms present in the unit cell after removing 2/3 of the face-centered atoms.
3. Initial Number of Face-Centered Atoms:
In an FCC structure, each face of the unit cell contains one atom. Since there are six faces, there are a total of 6 face-centered atoms in the unit cell.
4. Number of Face-Centered Atoms Removed:
If 2/3 of the face-centered atoms are removed, we need to calculate 2/3 of 6.
2/3 * 6 = 4
Thus, 4 face-centered atoms are removed from the unit cell.
5. Remaining Face-Centered Atoms:
After removing 4 face-centered atoms, we are left with 6 - 4 = 2 face-centered atoms in the unit cell.
6. Total Number of Atoms in the Unit Cell:
In addition to the face-centered atoms, there are also atoms at the eight corners of the unit cell. Each corner atom is shared between eight neighboring unit cells. Therefore, the number of corner atoms within the unit cell is 1/8.
Thus, the total number of atoms in the unit cell can be calculated as follows:
Number of corner atoms = 8 * 1/8 = 1
Number of face-centered atoms = 2
Total number of atoms = Number of corner atoms + Number of face-centered atoms = 1 + 2 = 3
7. Calculation of Occupied Space:
To calculate the percentage of occupied space, we need to consider the volume occupied by the atoms in the unit cell. In an FCC structure, each atom occupies 1/8th of the unit cell volume.
Percentage of occupied space = (Number of atoms * Volume occupied by each atom) / Total volume of the unit cell * 100%
Percentage of occupied space = (3 * 1/8) / 1 * 100% = 3/8 * 100% = 37%
Therefore, the correct answer is option 'D' (37%), which represents the percentage of occupied space in the unit cell after removing 2/3 of the face-centered atoms.
Free Test
FREE
| Start Free Test |
Community Answer
In a ccp structure, if 2/3 rd face-centred atoms are removed, then th...
In CCP (FCC) lattice,
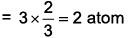
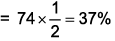
1 unit cell contain

Atoms removed from face centre
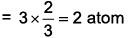
Since atoms become half so occupied space will become half that is
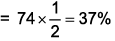

|
Explore Courses for NEET exam
|

|
Question Description
In a ccp structure, if 2/3 rd face-centred atoms are removed, then the percentage of occupied space in unit cell will bea)60%b)2.2%c)47.5 %d)37%Correct answer is option 'D'. Can you explain this answer? for NEET 2025 is part of NEET preparation. The Question and answers have been prepared according to the NEET exam syllabus. Information about In a ccp structure, if 2/3 rd face-centred atoms are removed, then the percentage of occupied space in unit cell will bea)60%b)2.2%c)47.5 %d)37%Correct answer is option 'D'. Can you explain this answer? covers all topics & solutions for NEET 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for In a ccp structure, if 2/3 rd face-centred atoms are removed, then the percentage of occupied space in unit cell will bea)60%b)2.2%c)47.5 %d)37%Correct answer is option 'D'. Can you explain this answer?.
In a ccp structure, if 2/3 rd face-centred atoms are removed, then the percentage of occupied space in unit cell will bea)60%b)2.2%c)47.5 %d)37%Correct answer is option 'D'. Can you explain this answer? for NEET 2025 is part of NEET preparation. The Question and answers have been prepared according to the NEET exam syllabus. Information about In a ccp structure, if 2/3 rd face-centred atoms are removed, then the percentage of occupied space in unit cell will bea)60%b)2.2%c)47.5 %d)37%Correct answer is option 'D'. Can you explain this answer? covers all topics & solutions for NEET 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for In a ccp structure, if 2/3 rd face-centred atoms are removed, then the percentage of occupied space in unit cell will bea)60%b)2.2%c)47.5 %d)37%Correct answer is option 'D'. Can you explain this answer?.
Solutions for In a ccp structure, if 2/3 rd face-centred atoms are removed, then the percentage of occupied space in unit cell will bea)60%b)2.2%c)47.5 %d)37%Correct answer is option 'D'. Can you explain this answer? in English & in Hindi are available as part of our courses for NEET.
Download more important topics, notes, lectures and mock test series for NEET Exam by signing up for free.
Here you can find the meaning of In a ccp structure, if 2/3 rd face-centred atoms are removed, then the percentage of occupied space in unit cell will bea)60%b)2.2%c)47.5 %d)37%Correct answer is option 'D'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
In a ccp structure, if 2/3 rd face-centred atoms are removed, then the percentage of occupied space in unit cell will bea)60%b)2.2%c)47.5 %d)37%Correct answer is option 'D'. Can you explain this answer?, a detailed solution for In a ccp structure, if 2/3 rd face-centred atoms are removed, then the percentage of occupied space in unit cell will bea)60%b)2.2%c)47.5 %d)37%Correct answer is option 'D'. Can you explain this answer? has been provided alongside types of In a ccp structure, if 2/3 rd face-centred atoms are removed, then the percentage of occupied space in unit cell will bea)60%b)2.2%c)47.5 %d)37%Correct answer is option 'D'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice In a ccp structure, if 2/3 rd face-centred atoms are removed, then the percentage of occupied space in unit cell will bea)60%b)2.2%c)47.5 %d)37%Correct answer is option 'D'. Can you explain this answer? tests, examples and also practice NEET tests.

|
Explore Courses for NEET exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















