Class 10 Exam > Class 10 Questions > name a non metal which conducts electricity
Start Learning for Free
name a non metal which conducts electricity
Most Upvoted Answer
name a non metal which conducts electricity
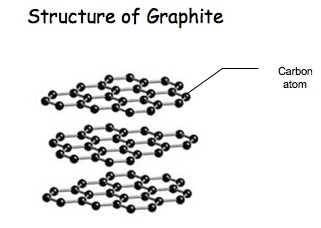
Graphite (/ˈɡræfaɪt/), archaically referred to as plumbago, is a crystalline form of the element carbon with its atoms arranged in a hexagonal structure. It occurs naturally in this form and is the most stable form of carbon under standard conditions. Under high pressures and temperatures it converts to diamond. Graphite is used in pencils and lubricants. It is a good conductor of heat and electricity. Its high conductivity makes it useful in electronic products such as electrodes, batteries, and somarksReferencColorIron-black to steel-gray; deep blue in transmitted lightCrystal habitTabular, six-sided foliated masses, granular to compacted massesTwinningPresentCleavageBasal – perfect on {0001}FractureFlaky, otherwise rough when not on cleavageTenacityFlexible non-elastic, sectileMohs scale hardness1–2LusterMetallic, earthyStreakBlackDiaphaneityOpaque, transparent only in extremely thin flakesSpecific gravity1.9–2.3Density2.09–2.23 g/cm3Optical propertiesUniaxial (–)PleochroismStrongSolubilitySoluble in molten nickel, warm chlorosulfuric acid[1]Other characteristicsstrongly anisotropic, conducts electricity, greasy feel, readily marks
Community Answer
name a non metal which conducts electricity
Attention Class 10 Students!
To make sure you are not studying endlessly, EduRev has designed Class 10 study material, with Structured Courses, Videos, & Test Series. Plus get personalized analysis, doubt solving and improvement plans to achieve a great score in Class 10.

|
Explore Courses for Class 10 exam
|

|
Similar Class 10 Doubts
name a non metal which conducts electricity
Question Description
name a non metal which conducts electricity for Class 10 2024 is part of Class 10 preparation. The Question and answers have been prepared according to the Class 10 exam syllabus. Information about name a non metal which conducts electricity covers all topics & solutions for Class 10 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for name a non metal which conducts electricity.
name a non metal which conducts electricity for Class 10 2024 is part of Class 10 preparation. The Question and answers have been prepared according to the Class 10 exam syllabus. Information about name a non metal which conducts electricity covers all topics & solutions for Class 10 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for name a non metal which conducts electricity.
Solutions for name a non metal which conducts electricity in English & in Hindi are available as part of our courses for Class 10.
Download more important topics, notes, lectures and mock test series for Class 10 Exam by signing up for free.
Here you can find the meaning of name a non metal which conducts electricity defined & explained in the simplest way possible. Besides giving the explanation of
name a non metal which conducts electricity, a detailed solution for name a non metal which conducts electricity has been provided alongside types of name a non metal which conducts electricity theory, EduRev gives you an
ample number of questions to practice name a non metal which conducts electricity tests, examples and also practice Class 10 tests.

|
Explore Courses for Class 10 exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.

























