NEET Exam > NEET Questions > The elements with atomic numbers 35, 53 and 8...
Start Learning for Free
The elements with atomic numbers 35, 53 and 85 are _________.
- a)noble gases
- b)halides
- c)alkaline
- d)halogens
Correct answer is option 'D'. Can you explain this answer?
Verified Answer
The elements with atomic numbers 35, 53 and 85 are _________.a)noble g...
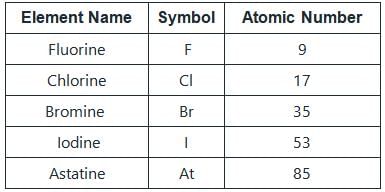
The Halogens are the elements belonging to Group 17 in the periodic table.
Most Upvoted Answer
The elements with atomic numbers 35, 53 and 85 are _________.a)noble g...
The correct answer is option 'D': halogens.
Halogens are a group of elements in the periodic table that belong to Group 17. They are known for their high reactivity and tendency to form salts. The three elements mentioned in the question, with atomic numbers 35, 53, and 85, are all halogens.
Let's analyze each element individually:
1. Element with atomic number 35:
The element with atomic number 35 is bromine (Br). Bromine is a reddish-brown liquid at room temperature and is the only nonmetallic element that exists in a liquid state. It belongs to the halogen family and is highly reactive. Bromine readily forms compounds with other elements, such as sodium bromide (NaBr) and hydrogen bromide (HBr).
2. Element with atomic number 53:
The element with atomic number 53 is iodine (I). Iodine is a solid at room temperature and appears as shiny purple-black crystals. Like other halogens, iodine is highly reactive and readily forms compounds. It is commonly used as an antiseptic and in the production of iodized salt.
3. Element with atomic number 85:
The element with atomic number 85 is astatine (At). Astatine is a highly radioactive element and is the rarest naturally occurring element on Earth. It is a halogen and exhibits similar chemical properties to the other halogens. Due to its radioactivity and short half-life, astatine is difficult to study and is mainly of scientific interest.
In summary, the elements with atomic numbers 35, 53, and 85 are bromine (Br), iodine (I), and astatine (At), respectively. These elements all belong to the halogen group in the periodic table, making option 'D' the correct answer.
Halogens are a group of elements in the periodic table that belong to Group 17. They are known for their high reactivity and tendency to form salts. The three elements mentioned in the question, with atomic numbers 35, 53, and 85, are all halogens.
Let's analyze each element individually:
1. Element with atomic number 35:
The element with atomic number 35 is bromine (Br). Bromine is a reddish-brown liquid at room temperature and is the only nonmetallic element that exists in a liquid state. It belongs to the halogen family and is highly reactive. Bromine readily forms compounds with other elements, such as sodium bromide (NaBr) and hydrogen bromide (HBr).
2. Element with atomic number 53:
The element with atomic number 53 is iodine (I). Iodine is a solid at room temperature and appears as shiny purple-black crystals. Like other halogens, iodine is highly reactive and readily forms compounds. It is commonly used as an antiseptic and in the production of iodized salt.
3. Element with atomic number 85:
The element with atomic number 85 is astatine (At). Astatine is a highly radioactive element and is the rarest naturally occurring element on Earth. It is a halogen and exhibits similar chemical properties to the other halogens. Due to its radioactivity and short half-life, astatine is difficult to study and is mainly of scientific interest.
In summary, the elements with atomic numbers 35, 53, and 85 are bromine (Br), iodine (I), and astatine (At), respectively. These elements all belong to the halogen group in the periodic table, making option 'D' the correct answer.

|
Explore Courses for NEET exam
|

|
Question Description
The elements with atomic numbers 35, 53 and 85 are _________.a)noble gasesb)halidesc)alkalined)halogensCorrect answer is option 'D'. Can you explain this answer? for NEET 2025 is part of NEET preparation. The Question and answers have been prepared according to the NEET exam syllabus. Information about The elements with atomic numbers 35, 53 and 85 are _________.a)noble gasesb)halidesc)alkalined)halogensCorrect answer is option 'D'. Can you explain this answer? covers all topics & solutions for NEET 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for The elements with atomic numbers 35, 53 and 85 are _________.a)noble gasesb)halidesc)alkalined)halogensCorrect answer is option 'D'. Can you explain this answer?.
The elements with atomic numbers 35, 53 and 85 are _________.a)noble gasesb)halidesc)alkalined)halogensCorrect answer is option 'D'. Can you explain this answer? for NEET 2025 is part of NEET preparation. The Question and answers have been prepared according to the NEET exam syllabus. Information about The elements with atomic numbers 35, 53 and 85 are _________.a)noble gasesb)halidesc)alkalined)halogensCorrect answer is option 'D'. Can you explain this answer? covers all topics & solutions for NEET 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for The elements with atomic numbers 35, 53 and 85 are _________.a)noble gasesb)halidesc)alkalined)halogensCorrect answer is option 'D'. Can you explain this answer?.
Solutions for The elements with atomic numbers 35, 53 and 85 are _________.a)noble gasesb)halidesc)alkalined)halogensCorrect answer is option 'D'. Can you explain this answer? in English & in Hindi are available as part of our courses for NEET.
Download more important topics, notes, lectures and mock test series for NEET Exam by signing up for free.
Here you can find the meaning of The elements with atomic numbers 35, 53 and 85 are _________.a)noble gasesb)halidesc)alkalined)halogensCorrect answer is option 'D'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
The elements with atomic numbers 35, 53 and 85 are _________.a)noble gasesb)halidesc)alkalined)halogensCorrect answer is option 'D'. Can you explain this answer?, a detailed solution for The elements with atomic numbers 35, 53 and 85 are _________.a)noble gasesb)halidesc)alkalined)halogensCorrect answer is option 'D'. Can you explain this answer? has been provided alongside types of The elements with atomic numbers 35, 53 and 85 are _________.a)noble gasesb)halidesc)alkalined)halogensCorrect answer is option 'D'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice The elements with atomic numbers 35, 53 and 85 are _________.a)noble gasesb)halidesc)alkalined)halogensCorrect answer is option 'D'. Can you explain this answer? tests, examples and also practice NEET tests.

|
Explore Courses for NEET exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















