JEE Exam > JEE Questions > When 2-butyne is treated with H2/Lindlars cat...
Start Learning for Free
When 2-butyne is treated with H2/Lindlar's catalyst, compound X is produced as the major product, and when treated with Na/liq. NH3, it produces y as the major product. Which of the following statements is correct?
- a)X will have higher dipole moment and higher boiling point than Y.
- b)Y will have higher dipole moment and lower boiling point than X.
- c)Y will have higher dipole moment and higher boiling point than X.
- d)X will have lower dipole moment and lower boiling point than Y.
Correct answer is option 'A'. Can you explain this answer?
Verified Answer
When 2-butyne is treated with H2/Lindlars catalyst, compound X is prod...
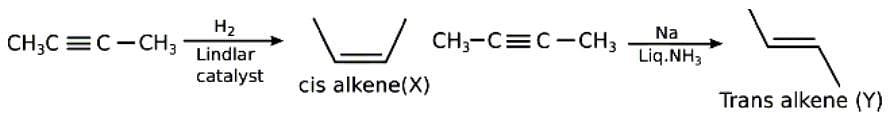
Dipole moment of X is more than Y.
Boiling point of X is more than Y.
Most Upvoted Answer
When 2-butyne is treated with H2/Lindlars catalyst, compound X is prod...
Answer:
Introduction:
The given question involves the reaction of 2-butyne with two different reagents, H2/Lindlars catalyst and Na/liq. NH3. We need to determine the major products formed in each case and compare their dipole moments and boiling points.
Reaction with H2/Lindlars catalyst:
When 2-butyne is treated with H2/Lindlars catalyst, it undergoes hydrogenation to produce compound X as the major product. The Lindlars catalyst is a poisoned catalyst that selectively reduces alkynes to cis-alkenes without further reduction to alkanes.
Reaction with Na/liq. NH3:
When 2-butyne is treated with Na/liq. NH3, it undergoes a reaction known as dissolving metal reduction. The sodium metal dissolves in liquid ammonia to form the deep blue color sodium-ammonia solution, which acts as a strong reducing agent. In this reaction, the triple bond of 2-butyne is reduced to form compound Y as the major product.
Comparison between compound X and Y:
To determine the dipole moment and boiling point of compounds X and Y, we need to analyze their structures.
Compound X:
Compound X is the cis-alkene formed by the hydrogenation of 2-butyne. It has the following structure:
H
|
H - C = C - C - H
|
H
The dipole moment of compound X is expected to be low since it is a symmetric molecule with no significant charge separation. The boiling point of compound X will be determined by intermolecular forces such as London dispersion forces, which are generally weaker for alkenes compared to alcohols or carboxylic acids.
Compound Y:
Compound Y is the product of dissolving metal reduction of 2-butyne. It has the following structure:
H
|
H - C = C - C - H
|
Na
The presence of the metal atom (Na) in compound Y can lead to a higher dipole moment compared to compound X. The metal atom can induce partial charges on the adjacent carbon atoms, resulting in a higher dipole moment. The boiling point of compound Y will also be determined by intermolecular forces, and the presence of the metal atom may influence the strength of these forces.
Conclusion:
Based on the analysis, the correct statement is option 'A': X will have a higher dipole moment and higher boiling point than Y. Compound X is a cis-alkene formed by hydrogenation, which is a symmetrical molecule with a low dipole moment. Compound Y is formed by dissolving metal reduction and contains a metal atom, which can lead to a higher dipole moment. Additionally, the boiling point of compound X is expected to be higher due to stronger intermolecular forces compared to compound Y.
Introduction:
The given question involves the reaction of 2-butyne with two different reagents, H2/Lindlars catalyst and Na/liq. NH3. We need to determine the major products formed in each case and compare their dipole moments and boiling points.
Reaction with H2/Lindlars catalyst:
When 2-butyne is treated with H2/Lindlars catalyst, it undergoes hydrogenation to produce compound X as the major product. The Lindlars catalyst is a poisoned catalyst that selectively reduces alkynes to cis-alkenes without further reduction to alkanes.
Reaction with Na/liq. NH3:
When 2-butyne is treated with Na/liq. NH3, it undergoes a reaction known as dissolving metal reduction. The sodium metal dissolves in liquid ammonia to form the deep blue color sodium-ammonia solution, which acts as a strong reducing agent. In this reaction, the triple bond of 2-butyne is reduced to form compound Y as the major product.
Comparison between compound X and Y:
To determine the dipole moment and boiling point of compounds X and Y, we need to analyze their structures.
Compound X:
Compound X is the cis-alkene formed by the hydrogenation of 2-butyne. It has the following structure:
H
|
H - C = C - C - H
|
H
The dipole moment of compound X is expected to be low since it is a symmetric molecule with no significant charge separation. The boiling point of compound X will be determined by intermolecular forces such as London dispersion forces, which are generally weaker for alkenes compared to alcohols or carboxylic acids.
Compound Y:
Compound Y is the product of dissolving metal reduction of 2-butyne. It has the following structure:
H
|
H - C = C - C - H
|
Na
The presence of the metal atom (Na) in compound Y can lead to a higher dipole moment compared to compound X. The metal atom can induce partial charges on the adjacent carbon atoms, resulting in a higher dipole moment. The boiling point of compound Y will also be determined by intermolecular forces, and the presence of the metal atom may influence the strength of these forces.
Conclusion:
Based on the analysis, the correct statement is option 'A': X will have a higher dipole moment and higher boiling point than Y. Compound X is a cis-alkene formed by hydrogenation, which is a symmetrical molecule with a low dipole moment. Compound Y is formed by dissolving metal reduction and contains a metal atom, which can lead to a higher dipole moment. Additionally, the boiling point of compound X is expected to be higher due to stronger intermolecular forces compared to compound Y.

|
Explore Courses for JEE exam
|

|
Similar JEE Doubts
When 2-butyne is treated with H2/Lindlars catalyst, compound X is produced as the major product, and when treated with Na/liq. NH3, it produces y as the major product. Which of the following statements is correct?a)X will have higher dipole moment and higher boiling point than Y.b)Y will have higher dipole moment and lower boiling point than X.c)Y will have higher dipole moment and higher boiling point than X.d)X will have lower dipole moment and lower boiling point than Y.Correct answer is option 'A'. Can you explain this answer?
Question Description
When 2-butyne is treated with H2/Lindlars catalyst, compound X is produced as the major product, and when treated with Na/liq. NH3, it produces y as the major product. Which of the following statements is correct?a)X will have higher dipole moment and higher boiling point than Y.b)Y will have higher dipole moment and lower boiling point than X.c)Y will have higher dipole moment and higher boiling point than X.d)X will have lower dipole moment and lower boiling point than Y.Correct answer is option 'A'. Can you explain this answer? for JEE 2025 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about When 2-butyne is treated with H2/Lindlars catalyst, compound X is produced as the major product, and when treated with Na/liq. NH3, it produces y as the major product. Which of the following statements is correct?a)X will have higher dipole moment and higher boiling point than Y.b)Y will have higher dipole moment and lower boiling point than X.c)Y will have higher dipole moment and higher boiling point than X.d)X will have lower dipole moment and lower boiling point than Y.Correct answer is option 'A'. Can you explain this answer? covers all topics & solutions for JEE 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for When 2-butyne is treated with H2/Lindlars catalyst, compound X is produced as the major product, and when treated with Na/liq. NH3, it produces y as the major product. Which of the following statements is correct?a)X will have higher dipole moment and higher boiling point than Y.b)Y will have higher dipole moment and lower boiling point than X.c)Y will have higher dipole moment and higher boiling point than X.d)X will have lower dipole moment and lower boiling point than Y.Correct answer is option 'A'. Can you explain this answer?.
When 2-butyne is treated with H2/Lindlars catalyst, compound X is produced as the major product, and when treated with Na/liq. NH3, it produces y as the major product. Which of the following statements is correct?a)X will have higher dipole moment and higher boiling point than Y.b)Y will have higher dipole moment and lower boiling point than X.c)Y will have higher dipole moment and higher boiling point than X.d)X will have lower dipole moment and lower boiling point than Y.Correct answer is option 'A'. Can you explain this answer? for JEE 2025 is part of JEE preparation. The Question and answers have been prepared according to the JEE exam syllabus. Information about When 2-butyne is treated with H2/Lindlars catalyst, compound X is produced as the major product, and when treated with Na/liq. NH3, it produces y as the major product. Which of the following statements is correct?a)X will have higher dipole moment and higher boiling point than Y.b)Y will have higher dipole moment and lower boiling point than X.c)Y will have higher dipole moment and higher boiling point than X.d)X will have lower dipole moment and lower boiling point than Y.Correct answer is option 'A'. Can you explain this answer? covers all topics & solutions for JEE 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for When 2-butyne is treated with H2/Lindlars catalyst, compound X is produced as the major product, and when treated with Na/liq. NH3, it produces y as the major product. Which of the following statements is correct?a)X will have higher dipole moment and higher boiling point than Y.b)Y will have higher dipole moment and lower boiling point than X.c)Y will have higher dipole moment and higher boiling point than X.d)X will have lower dipole moment and lower boiling point than Y.Correct answer is option 'A'. Can you explain this answer?.
Solutions for When 2-butyne is treated with H2/Lindlars catalyst, compound X is produced as the major product, and when treated with Na/liq. NH3, it produces y as the major product. Which of the following statements is correct?a)X will have higher dipole moment and higher boiling point than Y.b)Y will have higher dipole moment and lower boiling point than X.c)Y will have higher dipole moment and higher boiling point than X.d)X will have lower dipole moment and lower boiling point than Y.Correct answer is option 'A'. Can you explain this answer? in English & in Hindi are available as part of our courses for JEE.
Download more important topics, notes, lectures and mock test series for JEE Exam by signing up for free.
Here you can find the meaning of When 2-butyne is treated with H2/Lindlars catalyst, compound X is produced as the major product, and when treated with Na/liq. NH3, it produces y as the major product. Which of the following statements is correct?a)X will have higher dipole moment and higher boiling point than Y.b)Y will have higher dipole moment and lower boiling point than X.c)Y will have higher dipole moment and higher boiling point than X.d)X will have lower dipole moment and lower boiling point than Y.Correct answer is option 'A'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
When 2-butyne is treated with H2/Lindlars catalyst, compound X is produced as the major product, and when treated with Na/liq. NH3, it produces y as the major product. Which of the following statements is correct?a)X will have higher dipole moment and higher boiling point than Y.b)Y will have higher dipole moment and lower boiling point than X.c)Y will have higher dipole moment and higher boiling point than X.d)X will have lower dipole moment and lower boiling point than Y.Correct answer is option 'A'. Can you explain this answer?, a detailed solution for When 2-butyne is treated with H2/Lindlars catalyst, compound X is produced as the major product, and when treated with Na/liq. NH3, it produces y as the major product. Which of the following statements is correct?a)X will have higher dipole moment and higher boiling point than Y.b)Y will have higher dipole moment and lower boiling point than X.c)Y will have higher dipole moment and higher boiling point than X.d)X will have lower dipole moment and lower boiling point than Y.Correct answer is option 'A'. Can you explain this answer? has been provided alongside types of When 2-butyne is treated with H2/Lindlars catalyst, compound X is produced as the major product, and when treated with Na/liq. NH3, it produces y as the major product. Which of the following statements is correct?a)X will have higher dipole moment and higher boiling point than Y.b)Y will have higher dipole moment and lower boiling point than X.c)Y will have higher dipole moment and higher boiling point than X.d)X will have lower dipole moment and lower boiling point than Y.Correct answer is option 'A'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice When 2-butyne is treated with H2/Lindlars catalyst, compound X is produced as the major product, and when treated with Na/liq. NH3, it produces y as the major product. Which of the following statements is correct?a)X will have higher dipole moment and higher boiling point than Y.b)Y will have higher dipole moment and lower boiling point than X.c)Y will have higher dipole moment and higher boiling point than X.d)X will have lower dipole moment and lower boiling point than Y.Correct answer is option 'A'. Can you explain this answer? tests, examples and also practice JEE tests.

|
Explore Courses for JEE exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


























