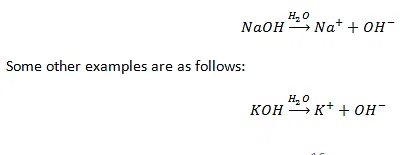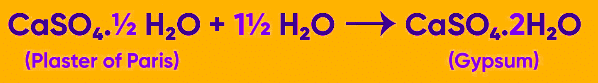Imagine you're enjoying a delicious meal, but later you feel that familiar burn of acidity in your stomach. What do you reach for—lemon juice, vinegar, or baking soda solution? Your mother probably suggests the baking soda solution to balance out the excess acid, you want to know why? we will read in this chapter the different reactions take place involving acid, base and salt.
Think about the last time you spilled curry on a white shirt. Did you notice how the stain turned reddish-brown when you scrubbed it with soap? That’s because soap is basic, and it reacts with the turmeric in the curry. Then, as you rinse it with water, the stain fades back to yellow.
You’ve learned that acids are sour, like lemon juice while bases are bitter. But how can we identify them without tasting, Indicators help us identify acids and bases without tasting them. In this chapter, we'll dive deeper into how acids and bases react with each other, and explore everyday examples like these that demonstrate their fascinating chemistry.
Understanding The Chemical Properties of Acids and Bases
(a) Acids and bases in laboratory
Let's us first know the basic of acids and bases
What is an Acid?
It is defined as a chemical compound that has a distinct sour flavor and possesses the property of being acidic. Chemically, it is characterized by a pH value that is less than 7, indicating that it has a higher concentration of hydrogen ions than hydroxide ions.
(i) Strong Acids: An acid, which dissociates completely or almost completely in water are strong acids. Examples: HCl, H2SO4, and HNO3.
Also called Inorganic acids except Carbonic acid (H2CO3) it is actually weak acid but inorganic.
(ii) Weak Acids: Acid that dissociates only partially when dissolved in water are weak acids. Examples: CH3COOH, Oxalic acid, and Lactic acid. Also called Organic acids.
(iii) Concentrated Acids: A concentrated solution is a liquid with a high solute concentration. A dilute acid is defined as a liquid with a lower solute concentration, while a concentrated acid has a higher solute concentration. (More amount of acid + Less amount of water). Examples: Conc. H2SO4 and Conc. HCl
(iv) Dilute Acids: A dilute solution is a liquid having a lower solute content. (More amount of water + Less amount of acid). Examples: H2SO4 with a concentration of 5% is considered a dilute acid.
What is Base?
A chemical compound that has a distinct bitter taste and possesses the property of being basic is defined as a base. Chemically, it is characterized by a pH value that is more than 7, indicating that it has a higher concentration of hydroxide ions than hydrogen ions.
Categories of Bases
These are as follows:
(i) Strong Bases: Strong bases are those which ionize in water completely and produce a large number of hydroxide ions. Weak bases are those bases that partially ionize in water and produce a small number of hydroxide ions.
Example: NaOH, KOH, Ca(OH)2
(ii) Weak Bases: Weak bases are those bases that partially ionize in water and produce a small amount of hydroxide ions.
Example: NH4OH
(iii) Alkalis: These are bases that are soluble in water.
Example: NaOH, KOH, Ca(OH)2.
Question for Chapter Notes: Acids, Bases & Salts
Try yourself:Which of the following chemical compounds has a sour taste and a pH value less than 7?
Explanation
To determine which chemical compound has a sour taste and a pH value less than 7, we need to recall some basic concepts:
-
Sour Taste: Acids are known for their sour taste. Common examples include vinegar (which contains acetic acid) and lemon juice (which contains citric acid).
-
pH Value: The pH scale measures how acidic or basic a substance is. A pH value less than 7 indicates an acidic substance, while a pH value greater than 7 indicates a basic substance.
Given this information, acetic acid (found in vinegar) is an acid, which means it has a sour taste and a pH value less than 7. Therefore, the correct answer is acetic acid. Option B
Report a problem
Now let's understand how to distinguish acid and base with help of indicator.
An indicator is a chemical compound that changes its colour in presence of an acid or base. Indicators are generally derived from plant pigments and are mildly acidic or basic in nature.
Activity 1:- Testing Laboratory Solutions with Indicators:
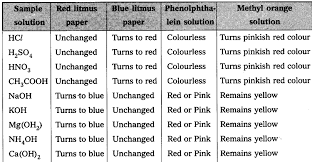
- Materials: Hydrochloric acid (HCl), sulfuric acid (H₂SO₄), nitric acid (HNO₃), acetic acid (CH₃COOH), sodium hydroxide (NaOH), calcium hydroxide (Ca(OH)₂), potassium hydroxide (KOH), magnesium hydroxide (Mg(OH)₂), ammonium hydroxide (NH₄OH), red and blue litmus paper, phenolphthalein, methyl orange.
- Procedure: Place a drop of each solution on a watch glass and test with red and blue litmus paper, phenolphthalein, and methyl orange.
- Observation: Record the color changes with each indicator for each solution to identify their acidic or basic nature.
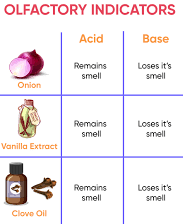
Activity 2:- Testing Olfactory Indicators:
- Materials: Finely chopped onions, a plastic bag, clean cloth strips, dilute HCl, dilute NaOH, vanilla essence, clove oil.
- Procedure:
- Place the chopped onions and cloth strips in a plastic bag, seal it, and leave it in the fridge overnight.
- After a day, take out the cloth strips, check their odor. After that, apply a few drops of dilute HCl to one strip and dilute NaOH to the other. Rinse both strips with water and note any changes in odor.
- Test the odor of vanilla essence and clove oil by applying dilute HCl and NaOH solutions, then observe any changes.
- Result: Determine which substances (onion, vanilla essence, clove oil) serve as effective olfactory indicators based on their odor changes.
Question for Chapter Notes: Acids, Bases & Salts
Try yourself:Which of the following is an example of an olfactory indicator?
Explanation
An olfactory indicator is a substance that changes its odor when mixed in an acidic or basic solution. The correct answer is option C) Vanilla extract, as it has a characteristic pleasant smell, which changes when it comes in contact with an acidic or basic solution.
Report a problem
Next Steps: We have learnt how to identify acid and base with indicators, now we will learn about their properties and reactions.
(b) How do Acids and Bases React with Metals?
In general, acids react with metals to give salt and release hydrogen gas. Bases generally don't react with metals,but some metals can react with bases to form salts and hydrogen gas. This is because both metals and bases tend to give up electrons, and if both do, there's no preferred direction for the electrons to go, so no reaction occurs. However, some metals, like zinc, are amphoteric and can react with both acids and bases. For example, when sodium hydroxide solution is heated with zinc, it forms sodium zincate and hydrogen gas
(i) Reaction of Acids with Metals
When an acid reacts with a metal, the metal undergoes a
displacement reaction, where it displaces hydrogen from the acid. This results in the formation of a
salt and hydrogen gas.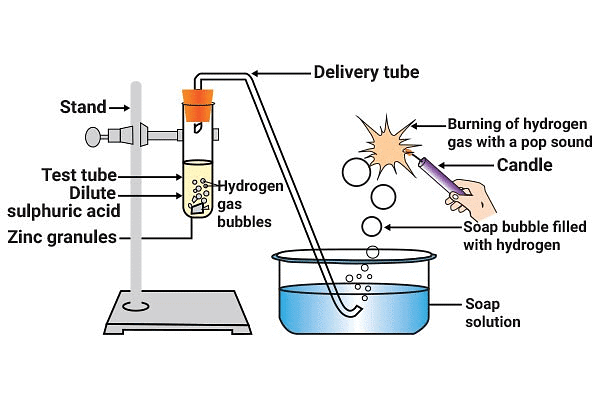 Reaction of Acids with Metals
Reaction of Acids with Metals
Equations like
Acid + Metal → Salt + Hydrogen Gas
Example: Zinc granules react with dilute Hydrochloric acid in a test tube.
HCl + Zn → ZnCl2 + H2↑
(ii) Reaction of Bases with Metals
Bases react with the metal to evolve hydrogen Gas. Also, note that all metals do not react with bases. The metal must be more reactive than the metals present in the base for the reaction to take place.
Equations like
Base + Metal → Salt + Hydrogen gas
Example: Zinc granules react with NaOH solution to form sodium zincate and evolve hydrogen gas.
2NaOH + Zn → Na2ZnO2 + H2↑
Note: Hydrogen gas released can be tested by bringing burning candle near gas bubbles, it burst with pop sound.
(c) How do Metal Carbonates and Metal Hydrogencarbonates React with Acids?

- Acids react with Metal Carbonates and Metal Hydrogen carbonates to form Salt, Carbon dioxide and water.
- Reaction: Metal carbonate/Metal hydrogen carbonate + Acid → Salt + Carbon dioxide + Water.
Examples:
(i) 2HCl + Na2CO3 → 2NaCl + CO2 + H2O
(ii) HCl + NaHCO3 → NaCl + CO2 + H2O - CO2 can be tested by passing it through lime water. It turns lime water milky.
Ca(OH)2 + CO2 → CaCO3 + H2O
Ca(OH)2- lime water CaCO3 - white precipitate
- When excess CO2 is passed, milkiness disappears.
CaCO3 + CO2 + H2O → Ca(HCO3)2 (aq)
Limestone, chalk and marble are different forms of calcium carbonate. All metal carbonates and hydrogen carbonates react with acids to give a corresponding salt, carbon dioxide and water. Thus, the reaction can be summarised as-
Metal carbonate/Metal hydrogencarbonate + Acid → Salt + Carbon dioxide + Water
- Bases do not react with Metal Carbonates and Metal Hydrogen carbonates.
Base + Metal Carbonate/Metal Hydrogen Carbonate → No Reaction
(d) How do Acids and Bases React with each other?
The reaction between an acid and a base is known as a neutralization reaction. When an acid and a base are mixed together, they react to form a salt and water.
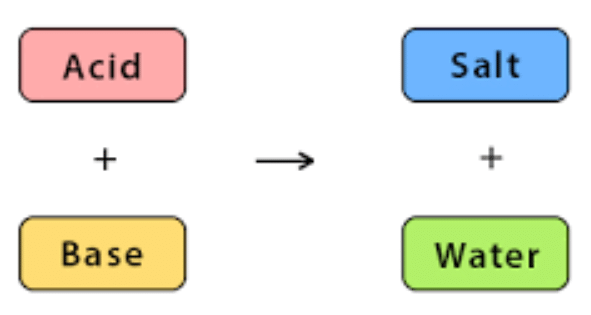
Reactions:
- Acids and Bases react to form salt and water.
Acid + Base → Salt + H2O - Neutralization Reaction: Reaction of acid with a base is called a neutralization reaction.
Example: HCl + NaOH → NaCl + H2O
Some more Reactions:
1. Strong Acid + Weak Base → Acidic salt + H2O
For example: HCl + NH3 → NH4Cl + H2O
(Hydrochloric acid + Ammonia → Ammonium chloride + Water)
2. Weak Acid + Strong Base → Basic salt + H2O
For example: CH3COOH + NaOH → CH3COONa + H2O
(Acetic acid + Sodium hydroxide → Sodium acetate + Water)
3. Strong Acid + Strong Base → Neutral salt + H2O
For example: H2SO4 + 2NaOH → Na2SO4 + 2H2O
(Sulfuric acid + Sodium hydroxide → Sodium sulfate + Water)
4. Weak Acid + Weak Base → Neutral salt + H2O
For example: CH3COOH + NH3 → CH3COONH4 + H2O
(Acetic acid + Ammonia → Ammonium acetate + Water)
Note:The pH of the resulting solution depends on the strength of the acid and base used. If the acid is strong and the base is weak, the resulting solution will be acidic. If the base is strong and the acid is weak, the resulting solution will be basic. If both the acid and base are equally strong or weak, the resulting solution will be neutral.
Question for Chapter Notes: Acids, Bases & Salts
Try yourself:What is the result of a neutralization reaction between an acid and a base?
Explanation
The correct answer is C) The acid and base react to form a salt and water.
- When an acid and a base are mixed together, they undergo a neutralization reaction, which results in the formation of a salt and water.
- The salt that is formed is typically composed of the cation from the base and the anion from the acid.
- This reaction is a common method used to neutralize acidic and basic solutions and is widely used in various applications, including in the production of fertilizers, pharmaceuticals, and cleaning products.
Report a problem
(e) Reaction of Metallic Oxides with Acids
(i) Reaction of Metallic Oxides with Acids
When a metallic oxide reacts with an acid, it typically produces salt and water.
The general chemical equation for this type of reaction is :
Metallic oxide + Acid → Salt + Water
Example: When calcium oxide (CaO) reacts with hydrochloric acid (HCl), it produces calcium chloride (CaCl2) and water (H2O):
CaO + 2HCl → CaCl2 + H2O
Since metallic oxides react with acids to give salts and water, similar to the reaction of a base with an acid, metallic oxides are primarily basic oxides, but some can also exhibit amphoteric properties.
(f) Reaction of Non-metallic Oxides with Bases
Non-metallic oxides are acidic and react with bases to form salt and water. The general chemical equation for the reaction between a non-metallic oxide and a base is:
Non-metallic oxide + Base → Salt + Water
The reaction between sulfur dioxide (SO2) and sodium hydroxide (NaOH):
SO2 + 2NaOH → Na2SO3+ H2O
- Non-metallic oxides are acidic in nature.
- Non-metallic Oxide + Base → Salt + H2O
CO2 + Ca(OH)2 → CaCO3 + H2O
Some Reactions of Acid:
(i) Acid + Metal Carbonate → Salt + CO2 + Water
(ii) Acid + Metal → Salt + H2
(iii) Acid + Metal Hydrogen Carbonate → Salt + CO2 + H2O
(iv) Acid + Metallic oxide → Salt +H2O
(v) Acid + Base → Salt + H2O
Some Reaction of Base:
(i) Base + Metal → Salt + H2
(ii) Base + Metal Carbonate → No Reaction
(iii) Base + Metal Hydrogen Carbonate → No Reaction
(iv) Base + Acid → Salt + H2O
(v) Base + Non Metallic oxide → Salt + H2O
What do all Acids and Bases have in common?
Acids and bases are two types of substances that have different chemical properties. However, they do share some similarities:
- Both acids and bases can be classified as electrolytes. This means that they can conduct electricity when dissolved in water.
- Acids and bases can react with each other to form salts and water. This reaction is called a neutralization reaction.
- Acids and bases can change the colour of certain indicators. For example, acids turn litmus paper from blue to red, while bases turn it from red to blue.
- Both acids and bases can be strong or weak. The strength of an acid or base depends on how many hydrogen ions (H+) or hydroxide ions (OH-) it produces when dissolved in water.
- Acids and bases are important in many chemical reactions. For example, acids are used in batteries, while bases are used in cleaning agents.
Question for Chapter Notes: Acids, Bases & Salts
Try yourself:What property do acids and bases share in common?
Explanation
The correct answer is B) They both can conduct electricity when dissolved in water.
- Acids and bases are both classified as electrolytes, meaning they can conduct electricity when dissolved in water. This property is due to the presence of charged particles (ions) in their solutions.
- The other options are incorrect. While some acids can taste sour (such as vinegar), not all acids do, and bases typically have a bitter taste.
- The pH of an acid or base can vary, with acids having a pH less than 7 and bases having a pH greater than 7. Finally, while some acids can react with metals to form salts, this is not a property that is shared by all acids and bases.
Report a problem
(a)What Happens to an Acid or a Base in a Water Solution?
- Acids produce H+ ions in the presence of water
- H+ ions cannot exist alone, they exist as H3O+ (hydronium ions).
H+ + H2O → H3O+
HCl + H2O → H3O+ + Cl- - Bases when dissolved in water give OH− ions.
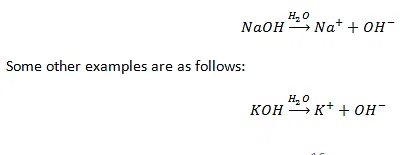
- Bases soluble in water are called alkalis.
- While diluting acids, it is recommended that the acid should be added to water, not the other way around because the process of dissolving an acid or a base in water is highly exothermic.
- Now as we have identified that all acids generate H+ (aq) and all bases generate OH– (aq), we can view the neutralisation reaction as follows – Acid + Base → Salt + Water
H X + M OH → MX + HOH
H+ (aq) + OH – (aq) → H2O(l)
Dissolving an acid or a base in water is a highly exothermic reaction, meaning it releases a lot of heat. When mixing concentrated nitric or sulfuric acid with water, it's crucial to add the acid slowly to the water while stirring continuously. Adding water to a concentrated acid can cause the mixture to become very hot, potentially causing it to splash and leading to burns. Additionally, the intense heat could even break the glass container.
When an acid or base is mixed with water, the concentration of ions (H₃O⁺/OH⁻) per unit volume decreases. This process is known as dilution, and the resulting solution is referred to as a diluted acid or base.
How Strong are Acid or Base Solutions?
The strength of an acid or base can be estimated using a
universal indicator.
- Universal indicator: It is a mixture of several indicators. It shows different colours at different concentrations of H+ ions in the solution.
- pH Scale: A scale for measuring H+ ion concentration in a solution. p in pH stands for ‘potenz’ a German word which means power.
- If the value of pH is equal to 7 → neutral solution
- If the value of pH is less than 7 → acidic solution
- If the value of pH is more than 7 → basic solution
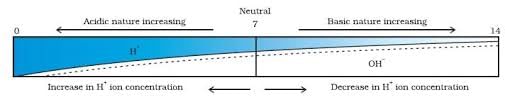 Variation of pH with the change in concentration of H+ (aq) and OH– (aq) ions
Variation of pH with the change in concentration of H+ (aq) and OH– (aq) ions
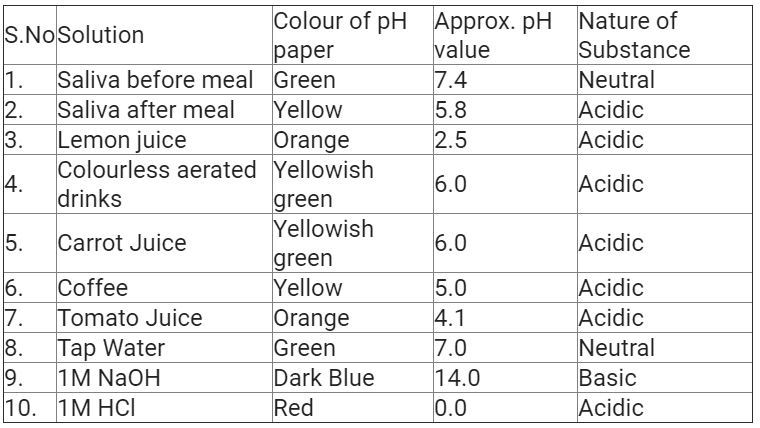
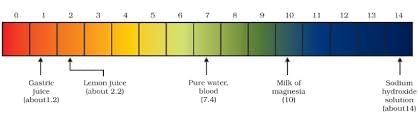 Image pH of some common substances shown on a pH paper (colours are only a rough guide)
Image pH of some common substances shown on a pH paper (colours are only a rough guide)
The strength of acids and bases depends on the number of H+ ions and OH– ions produced, respectively. If we take hydrochloric acid and acetic acid of the same concentration, say one molar, then these produce different amounts of hydrogen ions. Acids that give rise to more H+ ions are said to be strong acids, and acids that give less H+ ions are said to be weak acids. Strong bases produce more OH- in a solution because they fully accept protons.
Importance of pH in Everyday Life
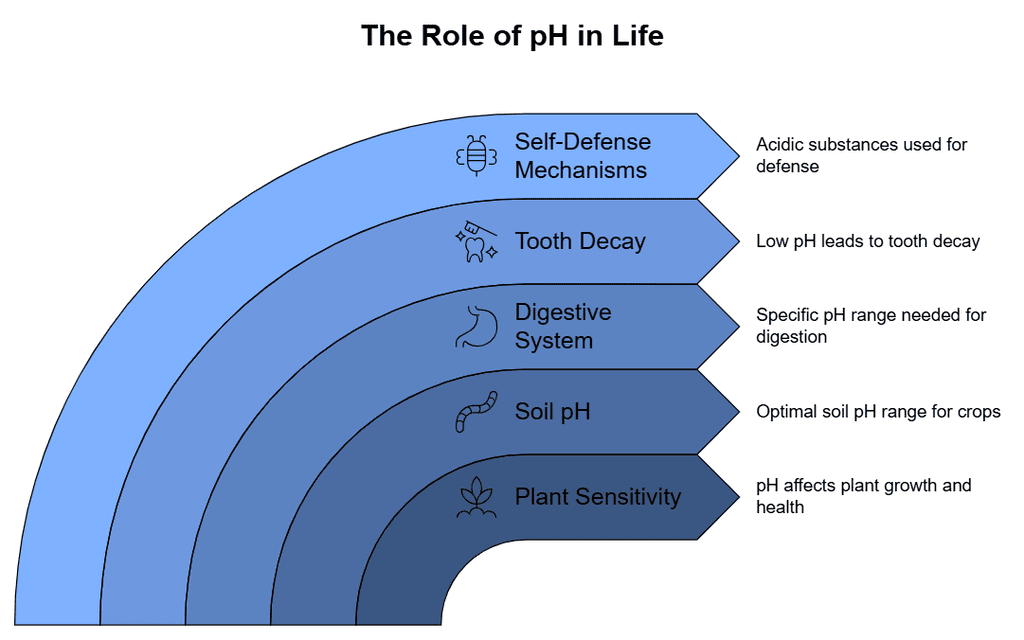
- Sensitivity of plants and animals: The pH level has a significant impact on both plants and animals. Many important biological processes, including enzyme and hormone activity as well as digestion, require a specific pH range to function properly. This means that even slight changes in pH can have a major impact on the overall health and well-being of living organisms.
- Soil: The ideal pH range for soil to support plant or crop growth is between 6.5 and 7.0.
- Digestive system: During the digestion process in our stomach, a particular pH range of 1.5 to 4 is required for optimal digestion to occur. The pH level of the enzymatic reactions that aid in the breakdown of food is affected by the presence of hydrochloric acid (HCl) in the stomach.
- Tooth decay: When the pH of the environment surrounding the teeth drops to 5.5 or lower, tooth decay can occur.
- Self-defense by animals and plants: Acidic substances are used by animals and plants as a self-defense mechanism. For example, bees and plants like nettle secrete a highly acidic substance for self-defense. These secreted acidic substances have a specific pH.
Nettle is a herbaceous plant which grows in the wild. Its leaves have stinging hair, which cause painful stings when touched accidentally. This is due to the methanoic acid secreted by them. A traditional remedy is rubbing the area with the leaf of the dock plant, which often grows beside the nettle in the wild.
More about Salts
It is defined as a substance that is odorless, salty in taste and soluble in water. Its pH value is equal to 7.
Examples:
- Sodium Chloride (NaCl) - It is commonly known as table salt.
- Epsom Salt (MgSO4) - It is used as a bath salt.
(a) Family of Salts
When salts have either the same cation or anion, they are categorized as members of the same family. For instance, salts like NaCl, KCl, and LiCl would be considered part of the same family because they have the same anion, which is chloride (Cl-). This concept of categorizing salts based on their shared cations or anions is important in chemistry and helps simplify the study of different salts.
(b) pH of Salts
- When a strong acid and a strong base combine to form a salt, the resulting compound will have a neutral pH of approximately 7.
- If a weak acid and a strong base combine to form a salt, the resulting compound will be basic with a pH greater than 7.
- Conversely, if a strong acid and a weak base combine to form a salt, the resulting compound will be acidic with a pH less than 7.
- The pH of a salt formed by a weak acid and a weak base cannot be predicted and must be determined through a pH test.
(c) Chemicals from Common Salt
Commonly known as salt, sodium chloride has a molecular formula of NaCl and is a fundamental element in our diet. It serves as a flavor enhancer and preservative in many of the foods we consume. From salt, it is possible to create four distinct compounds.
- Sodium hydroxide or lye or caustic soda
- Bleaching powder or calcium hypochlorite
- Baking soda or sodium hydrogen carbonate, or sodium bicarbonate
- Washing soda or sodium carbonate decahydrate
1. Sodium Hydroxide
When electricity is passed through an aqueous solution of sodium chloride (called brine), it decomposes to form sodium hydroxide. The process is called the chlor-alkali process because of the products formed– chlor for chlorine and alkali for sodium hydroxide.
2NaCl(aq) + 2H2O(l) → 2NaOH(aq) + Cl2 (g) + H2 (g)
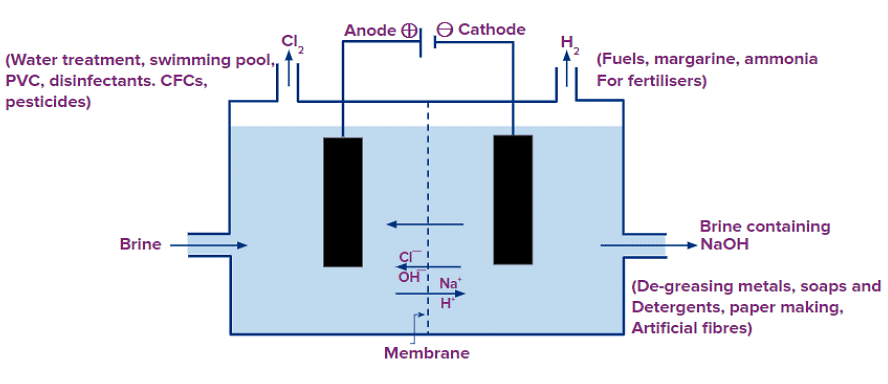
Electrolysis of brine (solution of common salt, NaCl) is carried out.
At anode: Cl2 is released
At cathode: H2 is released
Sodium hydroxide remains in the solution.
2. Bleaching Powder
Bleaching powder, which is produced by reacting chlorine gas with dry slaked lime (Ca(OH)2), is a water-soluble compound commonly used as a bleaching agent in the textile industry, as well as an oxidizing agent and disinfectant in various other industries. It should be noted that its primary use is as a bleaching agent, and it is made by a chemical reaction involving chlorine gas and Ca(OH)2.
Chemical formula – Ca(OCl)2 or CaOCl2
Preparation – Ca(OH)2(aq)+Cl2(g)→CaOCl2(aq)+H2O(l)
Uses of Bleaching Powder
- It finds application in the laundry for whitening soiled clothes and in the textile industry for bleaching cotton and linen due to its bleaching properties.
- Due to its strong oxidizing abilities, it serves as an oxidizer in several industries.
- It is utilized as a disinfectant to make water potable by eliminating harmful microorganisms.
3. Baking Soda
Sodium bicarbonate, also referred to as baking soda or bicarbonate of soda, is a type of chemical compound represented by the formula NaHCO3 and named sodium hydrogen carbonate by IUPAC. This compound is formed by the combination of a sodium cation (Na+) and a bicarbonate anion (HCO3). Typically, sodium bicarbonate appears as a fine white powder with a slightly salty and alkaline taste, similar to sodium carbonate or washing soda.
NaCl + H2O + CO2 + NH3 → NH4 Cl + NaHCO3
Chemical formula – NaHCO3
When it is heated during cooking:
2NaHCO3 → Na2CO3 + H2O + CO2
Sodium hydrogencarbonate has various uses in the household.
Uses of Baking Soda
- For making baking powder, which is a mixture of baking soda (sodium hydrogencarbonate) and a mild edible acid such as tartaric acid. When baking powder is heated or mixed in water, the following reaction takes place –
NaHCO3 + H+ → CO2 + H2O + Sodium salt of acid
(From any acid)
- Carbon dioxide produced during the reaction can cause bread or cake to rise making them soft and spongy.
- Sodium hydrogencarbonate is also an ingredient in antacids. Being alkaline, it neutralises excess acid in the stomach and provides relief.
- It is also used in soda-acid fire extinguishers.
4. Washing Soda
Sodium carbonate, also known as washing soda, is another chemical that can be derived from sodium chloride. As mentioned earlier, baking soda can be heated to produce sodium carbonate, which can then be recrystallized to yield washing soda in its hydrated form Na2CO3.10H2O. Similar to sodium carbonate, washing soda is also a basic salt with alkaline properties.
Na2CO3 + H2O → Na2CO3.10H2O
Uses of Washing Soda
- It finds applications in the glass, soap, and paper industries.
- The compound is utilized in the production of other sodium compounds like borax.
- Sodium carbonate serves as a cleaning agent for household use.
- It is employed to eliminate the problem of permanent water hardness.
(d) Are crystals are really dry?
When certain salts combine with a specific amount of water, they can form crystals. The water that combines with the salt is known as water of crystallization. Crystallization is the process in which a solid substance is formed, where the atoms or molecules are arranged in a strong structure called a crystal. This can occur through precipitation from a solution, freezing, or sometimes by direct deposition from a gas.
 Salt Crystals
Salt Crystals
Example: Table salt, also known as sodium chloride or halite crystals, sugar in the form of sucrose, and snowflakes are all familiar examples of materials that have crystalline structures. Similarly, numerous precious and semi-precious gemstones, including diamond and quartz, are classified as crystals due to their well-defined internal arrangement of atoms or molecules.
1. Copper sulfate crystals
Copper sulphate crystals which seem to be dry contain water of crystallisation. When we heat the crystals, this water is removed and the salt turns white. If you moisten the crystals again with water, you will find that blue colour of the crystals reappears.
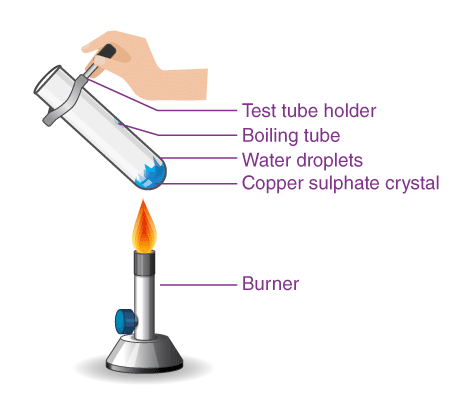 Removing water of crystallization
Removing water of crystallization Water of crystallization refers to the specific number of water molecules bound within each formula unit of a salt. For copper sulfate, there are five water molecules per formula unit, and its chemical formula is
CuSO₄·5H₂O.
This concept can help determine if the molecule of Na₂CO₃·10H₂O, washing soda is wet. Another example of a salt with water of crystallization is gypsum, which contains two water molecules in each formula unit. Its chemical formula is CaSO₄·2H₂O. Let's explore the uses of gypsum.
Question for Chapter Notes: Acids, Bases & Salts
Try yourself:What is the term for the water that combines with certain salts to form crystals?
Explanation
The correct answer is C) Water of crystallization. When certain salts combine with water, they can form crystals, and the water that combines with the salt is known as water of crystallization.
Report a problem
2. Plaster of Paris
Plaster of Paris is a commonly used chemical compound that finds wide application in sculpting materials and gauze bandages. It is essentially a white powder consisting of hydrated calcium sulfate, which is obtained by calcining gypsum. Although we encounter Plaster of Paris frequently in our daily lives, its chemical composition can be described as hydrated calcium sulfate, which is derived from gypsum that has been subjected to high temperatures during manufacturing.
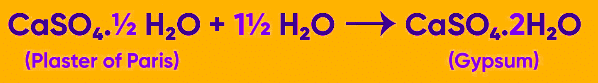
Gypsum plaster and the Plaster of Paris are essentially the same thing. The chemical formula for Plaster of Paris is CaSO4. ½ H2O. If gypsum (CaSO4.2H2O (s)) is heated to a temperature of 100°C (373K), it undergoes a reaction that yields CaSO4. ½ H2O and 3/2 H2O. Plaster of Paris is formed when the compound CaSO4. ½ H2O is produced. The chemical formula for this compound indicates that two units of CaSO4 share one molecule of water.
Uses of Plaster of Paris
- Medical casting: Plaster of Paris is often used in orthopedic medicine to create casts for broken bones. When mixed with water, it forms a paste that can be molded into the desired shape around the injured area. The plaster then hardens to create a sturdy cast that helps support the bone as it heals.
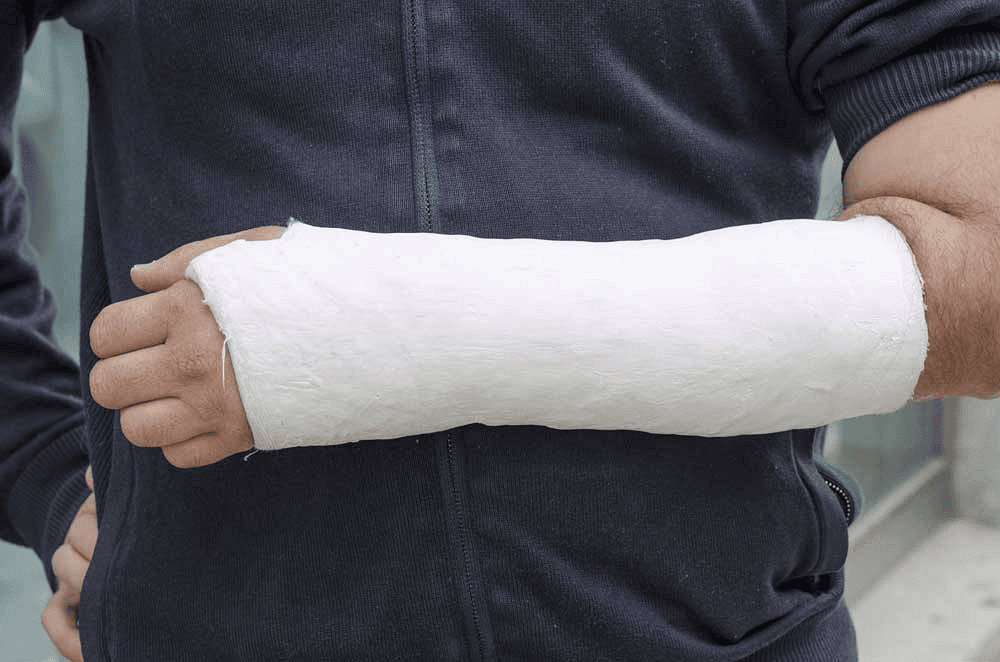 Use of Plaster of Paris
Use of Plaster of Paris
- Sculpting and art: Plaster of Paris is a popular material in sculpting and art. It can be molded into various shapes and textures and dries quickly, making it a convenient choice for creating intricate designs. Artists often use it to make casts, masks, and other decorative pieces.
- Home decoration: Plaster of Paris is also used for home decoration. It can be used to create decorative wall accents, ceiling tiles, and other architectural details. Plaster of Paris can also be used to make decorative figurines, ornaments, and other crafts. Its versatility and ease of use make it a popular choice for DIY projects.
Frequently Asked Questions (FAQs)
Q1. What is an acid?
Ans. An acid is a chemical substance that donates hydrogen ions (H+) or protons in a chemical reaction. Acids have a pH less than 7 and taste sour. Examples of acids are hydrochloric acid, sulfuric acid, and acetic acid.
Q2. What is a base?
Ans. A base is a chemical substance that accepts hydrogen ions (H+) or donates hydroxide ions (OH-) in a chemical reaction. Bases have a pH greater than 7 and taste bitter. Examples of bases are sodium hydroxide, potassium hydroxide, and calcium hydroxide.
Q3. What is a salt?
Ans. A salt is a chemical compound formed when an acid reacts with a base. It is made up of ions, which are formed when the acid and base neutralize each other. The most common salt is sodium chloride (table salt), which is formed when hydrochloric acid reacts with sodium hydroxide.
Q4. What is the pH scale?
Ans. The pH scale is a measure of the acidity or basicity of a solution. It ranges from 0 to 14, with 0 being the most acidic, 7 being neutral, and 14 being the most basic. The pH scale is logarithmic, which means that a change of one pH unit represents a ten-fold change in acidity or basicity.
Q5. What are some common uses of acids, bases, and salts?
Ans. Acids are commonly used in the production of fertilizers, dyes, and plastics. They are also used in the food industry to give tartness to certain foods. Bases are used in the production of soaps, detergents, and cleaning products. They are also used in agriculture to neutralize acidic soil. Salts are used in the food industry as preservatives and flavor enhancers. They are also used in the production of glass, ceramics, and metallurgy.