Carbon and Its Compounds Class 10 Notes Science Chapter 4
Carbon is an incredibly important element found in many things we use every day, like food, clothes, medicines, and books. Even though there's only a tiny bit of carbon in the Earth's crust (0.02%) and in the atmosphere (0.03% as carbon dioxide), it plays a huge role in our lives. This chapter will explore why carbon is so crucial and what makes it so special.

1. Bonding in Carbon - The Covalent Bond
Carbon compounds are substances made primarily of carbon atoms bonded with other elements. These compounds exhibit specific properties that distinguish them from other types of compounds.

Properties of Carbon Compounds:
- Poor Conductivity: Most carbon compounds do not conduct electricity well. This means they do not allow electric current to flow through them easily.
- Low Melting and Boiling Points: Carbon compounds generally have low melting and boiling points in comparison to ionic compounds. This indicates that the forces holding the molecules together are relatively weak.
- Weak Intermolecular Forces: The forces of attraction between molecules in carbon compounds are not very strong, leading to their low melting and boiling points.
- Non-Conductivity: Since carbon compounds are mostly non-conductors of electricity, it suggests that the bonding within these compounds does not result in the formation of ions.
Electron Distribution in Carbon
- Carbon has 6 electrons. These are distributed as 2 in the first shell and 4 in the second shell.
- Carbon has 4 valence electrons since these are the electrons in its outermost shell

Reactivity and Noble Gas Configuration:
- Elements aim to achieve a stable outer shell similar to noble gases.
- Carbon needs 4 more electrons to achieve a stable configuration
Note: Carbon can achieve stability by -
(a) Gaining Electrons:
- Carbon could gain 4 electrons to become C4- (anion).
- This would be challenging since the nucleus, with 6 protons, would struggle to hold onto 10 electrons.
(b) Losing Electrons:
- Carbon could lose 4 electrons to become C4+ (cation).
- This process would demand a lot of energy to remove 4 electrons, leaving behind a carbon cation with only 2 electrons.
Carbon solves this problem by bonding with other carbon atoms or with atoms of different elements through sharing its outer electrons. This sharing happens when atoms join together and share their outermost electrons. This sharing of electrons helps each atom achieve a stable configuration, similar to that of noble gases.
What is a Covalent Bond?
Covalent bonds are formed when two atoms share an electron pair, resulting in strong bonds within molecules but weak intermolecular forces
Properties of covalent Bond
- Covalent compounds have low melting and boiling points in general.
- In water and other polar solvents, covalent compounds are often insoluble or less soluble.
- When fused or dissolved, these materials are poor conductors of electricity.
Example:
(i) Hydrogen Molecule (H2)
- Hydrogen has an atomic number of 1, meaning it has one electron in its outer shell.
- To complete its shell, hydrogen needs one more electron.
- Two hydrogen atoms share their electrons to form a hydrogen molecule (H₂).
- This sharing allows each hydrogen atom to have the same electron configuration as helium, the nearest noble gas.

(ii) Oxygen Molecule ( O2)
- Oxygen forms a double bond between two oxygen atoms.
- Each oxygen atom has six electrons in its outer shell and needs two more to fill it.
- To complete their outer shells, the two oxygen atoms each share two electrons with each other.
- This sharing creates two pairs of shared electrons.
- The two pairs of shared electrons make up a double bond between the oxygen atoms.

(iii) Methane (CH4)
- It is one of the simplest compounds formed by carbon.
- The formula for methane is CH₄.
- Hydrogen has a valency of 1.
- Carbon has a valency of 4 because it has four outer electrons.
- To achieve a stable electron arrangement, carbon shares its four electrons with four hydrogen atoms.

There are several ways to generate a covalent bond.
(i) Single Covalent Bond: Formed by the sharing of one pair of electrons between two atoms.
Example: In HCl (hydrochloric acid), hydrogen shares one electron with chlorine.

(ii) Multiple Covalent Bonds: Involve the sharing of more than one pair of electrons.
- Double Bond: Shares two pairs of electrons.
- Triple Bond: Shares three pairs of electrons.
Example: In CO₂ (carbon dioxide), carbon forms two double bonds with two oxygen atoms.


Covalent Bonding in Hydrogen, Nitrogen and Water
The atoms of other elements like hydrogen, oxygen and nitrogen, chlorine also form bonds by sharing of electrons.
- Hydrogen is the simplest and most abundant element in the universe. It is also a very versatile element with a unique bonding capability.
- Hydrogen has only one electron in its valence shell, making it highly reactive and able to form strong bonds with many other elements. The most common type of bonding in hydrogen is covalent bonding, where it shares electrons with other elements to form molecules.
- Hydrogen can also form ionic bonds, where it donates or accepts electrons to form ions. These bonding properties make hydrogen a critical component in many chemical reactions, including those that power the sun and other stars.
 H-H Single bond between Hydrogen Atoms(H2)
H-H Single bond between Hydrogen Atoms(H2)
- Nitrogen is a vital element found in both organic and inorganic compounds, and its bonding properties are essential to its many applications.
- Nitrogen has five electrons in its outermost shell, which allows it to form a wide range of bonding arrangements, including covalent, ionic, and metallic bonds.
- Covalent bonding is the most common type of bond found in nitrogen-containing compounds, where nitrogen shares electrons with other nonmetal atoms to form stable molecules. These compounds have a wide range of applications, from fertilizers to pharmaceuticals
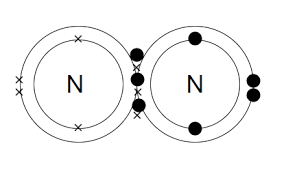 N≡ N Triple bond between Nitrogen atoms
N≡ N Triple bond between Nitrogen atoms
- Water is a unique and essential compound that plays a critical role in many aspects of life on Earth. The bonding in water is a key factor that gives it its distinctive properties.
- Water molecules consist of two hydrogen atoms covalently bonded to one oxygen atom, forming a V-shaped molecule.
- The hydrogen atoms are bonded to the oxygen atom by sharing electrons, resulting in a polar covalent bond. This means that the electrons in the bond are not shared equally, with the oxygen atom pulling the electrons closer to itself.
 The water molecule has a single covalent between one oxygen and two hydrogen atoms(H2O)
The water molecule has a single covalent between one oxygen and two hydrogen atoms(H2O)
1.1 Allotropes of Carbon
The property due to which an element exists in two or more physical forms is called Allotropy.
The different physical forms of an element are called Allotropes.
Carbon has the unique property of existing in different forms such as diamond, Graphite, Coke, Charcoal, Lampblack and gas carbon. Allotropes have different physical properties (Due to different arrangements of atoms in them but have the same chemical properties).
(a) Diamond - In Diamond, each carbon atom is bonded to four other carbon atoms, by four single covalent bonds, forming a rigid, three–dimensional, closely packed, structure. This results in a Higher density of Diamond (than Graphite). All valence electrons are used in bonding, Hence, no Free electrons - No electrical conductivity

(b) Graphite - In Graphite, each carbon atom is bonded to three other Carbon atoms in the same plane giving a hexagonal array (arrangement). One electron on each carbon atom is free (Not used in bonding). Hence, Graphite is a good conductor of electricity. Graphite structure is formed by the hexagonal array being Placed in layers one above the other, bonded by weak forces (Van der Waals Forces), hence the layers can slip above one another.

(c) Fullerenes - First one to be identified was C-60 which has carbon atoms arranged in the shape of a football. Since this looked like the geodesic dome designed by the US Architect Buck Minster Fuller, the Molecule was named Fullerene. Bucky Balls (C-60) were discovered in soot. They are as soft as Graphite. It consists of 20 Hexagons and 12 Pentagons. This was nicknamed as ‘Bulky Ball’.

2. Versatile Nature of Carbon
Carbon is a unique element in that it has two characteristic properties (Catenation and Tetravalency) that lead to the formation of a large number of compounds.

(i) Catenation: Self-Linking Ability of Carbon Atoms
- Carbon atoms possess the unique ability to form long chains or rings through covalent bonds.
- This allows carbon atoms to bond with other carbon atoms, resulting in linear, branched, or cyclic structures.
- The strong covalent bonds between carbon atoms enable the formation of stable molecules with diverse chemical and physical properties.

(ii) Tetravalency: Four Valence Electrons
- Carbon atoms have four valence electrons available for bonding.
- This tetravalency enables carbon to form strong covalent bonds with various atoms, including hydrogen, oxygen, nitrogen, and sulphur.
- Carbon can establish single, double, or triple bonds with these atoms, based on the number of available electrons and desired molecular stability.
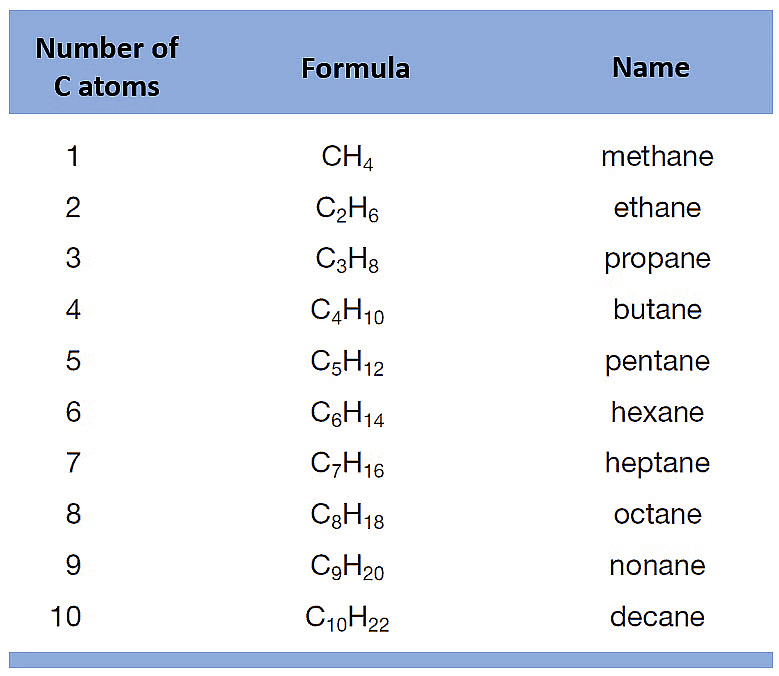
2.1 Saturated and Unsaturated Carbon Compounds
Compounds made up of hydrogen and carbon are called hydrocarbons.
There are two types of Hydrocarbons.

(i) Saturated Hydrocarbons
Saturated hydrocarbons are hydrocarbons that contain only single bonds between carbon atoms. They are the simplest class of hydrocarbons.
- Single bond between carbon atoms.
- ㅡCㅡCㅡ
- Alkanes are saturated hydrocarbons. General Formula: CnH2n+2
Example: Ethane C2H6


 Formulae and structures of saturated compounds
Formulae and structures of saturated compounds
(ii) Unsaturated Hydrocarbons
Unsaturated hydrocarbons are characterized by having at least one double or triple bond between carbon atoms. This means they have fewer hydrogen atoms compared to saturated hydrocarbons, which only have single bonds between carbon atoms.
- Double or triple bond between carbon atoms.
- Alkenes and Alkynes are unsaturated hydrocarbons.
- Alkenes: ㅡC=Cㅡ (General formula: CnH2n )
- Alkynes: ㅡC≡Cㅡ ( General Formula: CnH2n-2 )
Example: Ethene & Ethyne

 Formulae and structures of unsaturated compounds
Formulae and structures of unsaturated compounds
2.2 Chains, Branches, and Rings
Carbon atoms can form various structures, which significantly influence the properties of the molecules. These structures include straight chains, branched chains, and rings
(i) Straight (unbranched) chain
They have a linear arrangement of carbon atoms bonded together with single covalent bonds.
Example: C3H8

(ii) Branched Structure
These three above compounds have the same molecular formula but different structures called structural isomers and the phenomenon is structural isomerism.
Example: Pentane (C5H12)

Note: Isomers - Compounds having same molecular formula but different structures

(iii) Cyclic Structure
Cyclic hydrocarbons, also known as cyclic compounds, are organic molecules that contain carbon atoms arranged in a ring structure.

2.3 Will You Be My Friend
Carbon is a versatile element that can form bonds with various other elements like halogens, oxygen, nitrogen, and sulfur, besides hydrogen. When these elements replace hydrogen in a chain of hydrocarbons, they are called heteroatoms.
- These heteroatoms, found in specific groups, give unique properties to compounds, known as functional groups.
- Functional groups are crucial as they determine the characteristics of a compound, regardless of the length or type of the carbon chain they are attached to.
- These heteroatoms or group of atoms which make carbon compound reactive and decides their properties are called functional groups.

2.4 Homologous Series
Organic Compounds having the same General Molecular Formula, Similar structure and Similar Chemical Properties, placed together in the same group are called Homolog, and the series of such similar compounds is called Homologous series.
Characteristics of Homologous series
- All the members of a homologous series have the same general molecular formula.
- The difference in molecular formulae between any two adjacent members of a homologous series is CH2.
- The difference in molecular mass between any two adjacent members of a homologous series is 14.
- The methods of preparation and chemical properties of any member of the series are similar.
- The physical properties such as melting point, and boiling point in a homologous series change gradually as we go down the series.
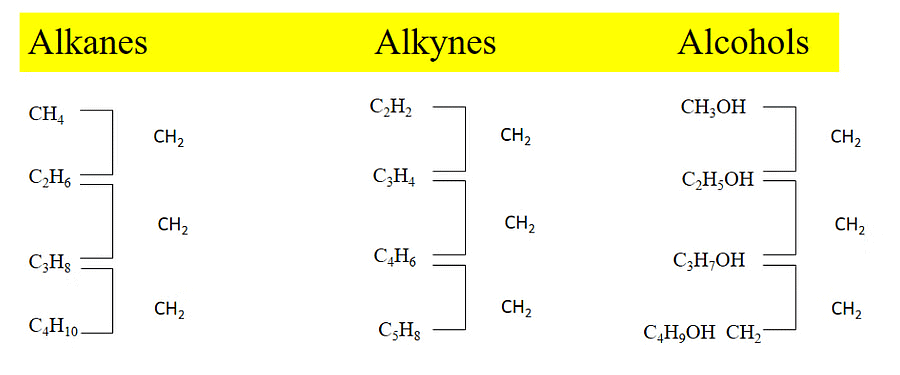 Homologous Series
Homologous Series
2.5 Nomenclature of Carbon Compounds
When naming a carbon compound, the number of carbon atoms in the compound is identified and the name of the basic carbon chain is modified by a prefix or a suffix indicating the nature of the functional group present in the compound.
- The functional group can be indicated by a prefix or a suffix. If the suffix of the functional group begins with a vowel, such as a, e, i, o, u, the final 'e' in the name of the carbon chain is removed and the appropriate suffix is added. For example, a three-carbon chain with a ketone group would be named as propanone.
- If the carbon chain is unsaturated, the final 'ane' in the name of the carbon chain is substituted by 'ene' or 'yne'.
- The names of compounds in a homologous series are based on the name of the basic carbon chain modified by a prefix or a suffix indicating the nature of the functional group.


3. Chemical Properties of Carbon Compounds
3.1 Combustion
- Carbon and its compounds are used as fuels because they burn in the air releasing a lot of heat energy.
- Saturated hydrocarbons generally burn in the air with blue and non-sooty flame.
- Unsaturated hydrocarbon burns in air with a yellow sooty flame because the percentage of carbon is higher than saturated hydrocarbon which does not get completely oxidized in the air.
- C + O2 → CO2 + heat and light
- CH4 + O2 → CO2 + H2O + heat and light
- CH3CH2OH + O2 → CO2 + H2O + heat and light
3.2 Oxidation
- Oxidation in carbon compounds refers to a chemical reaction in which the gain of oxygen or the loss of hydrogen from the carbon compound is , resulting in the formation of new functional groups.
- Alcohols can be converted to carboxylic acid in presence of oxidizing agent alkaline KMnO4 (potassium permanganate) or acidic potassium dichromate.

3.3 Addition Reaction
- Unsaturated hydrocarbon adds hydrogen in the presence of catalyst palladium or nickel.
- Vegetable oils are converted into vegetable ghee using this process.
- It is also called hydrogenation of vegetable oils.

3.4 Substitution Reaction
- The reaction between chlorine and alkane is an example of this halogenation process.
- When exposed to sunlight, the chlorine molecules dissociate into highly reactive chlorine radicals, which can abstract hydrogen atoms from the alkane, forming hydrochloric acid (HCl) and a hydrocarbon radical.
- This hydrocarbon radical can then react with another chlorine molecule, replacing the hydrogen atom with a chlorine atom and forming a chloroalkane.
- CH4 + Cl2 → CH3Cl + HCl (in the presence of sunlight)
4. Some Important Carbon Compounds: Ethanol or Ethyl Alcohol
4.1 Properties of Ethanol
Physical Properties
- Colourless, pleasant smell and burning taste.
- Soluble in water.
- Volatile liquid with a low boiling point of 351 K.
- Neutral compound.
Chemical Properties
(i) Reaction with Sodium
This reaction is used as a test for ethanol by the evolution of H2 gas (Burn with pop sound).
2Na + 2CH3CH2OH → 2CH3CH2O–Na+ + H2
(ii) Dehydration

4.2 Properties of Ethanoic Acid
Physical Properties
- Colourless liquid having sour taste and have smell of vinegar.
- Boiling point is 391 K.
- When pure CH3COOH is frozen, it forms a colourless ice-like solid. So it is called glacial acetic acid.
Chemical Properties
(i) Esterification

Sweet-smelling ester is formed

This is saponification as soap is prepared by this
(ii) Reaction with base
NaOH + CH3COOH → CH3COONa + H2O
(iii) Reaction with carbonates and hydrogen carbonates :
2CH3COOH + Na2CO3 → 2CH3COONa + H2O + CO2
CH3COOH + NaHCOH2 → CH3COONa + H2O + CO2
5. Soaps and Detergents
- Soap is sodium or potassium salt of long-chain carboxylic acid. Example: C17H35COONa+
- Soaps are effective only in soft water.
- Detergents are ammonium or sulphonate salt of long chain of carboxylic acid.
- Detergents are effective in both hard and soft water.
A soap molecule has:
(i) Ionic (hydrophilic) part
(ii) Long hydrocarbon chain (hydrophobic) part

Cleansing Action of Soap
Most dirt is oily and the hydrophobic end attaches itself to dirt and the ionic end is surrounded by molecules of water. This results in the formation of a radial structure called micelles. Soap micelles help to dissolve dirt and grease in water and the cloth gets cleaned.
 Micelle Formation
Micelle Formation
- The magnesium and calcium salt present in hard water reacts with soap molecules to form an insoluble product called scum. This scum creates difficulty in a cleansing action.
- By use of detergent, insoluble scum is not formed with hard water and cloths get cleaned effectively.
Synthetic Detergents:
- Detergents are effective non-soapy cleaning agents utilizing surface-active ingredients.
- Synthetic detergents, termed "soapless soaps," are efficient in hard or salt water and do not form scum.
- Modern synthetic detergents, such as alkyl or aryl sulphonates, are made from petroleum and sulfuric acid.
- Examples of Detergents: Two fundamental examples of well-known sulphonate or sulphate detergents are presented.
 Detergent
Detergent
Cleansing Action of Detergents:
- The molecular structure of synthetic detergents allows for a cleansing action similar to soaps, involving micelle formation and emulsification.
- Detergents lather effectively in hard water, dissolving calcium or magnesium ions.

|
80 videos|569 docs|80 tests
|
FAQs on Carbon and Its Compounds Class 10 Notes Science Chapter 4
| 1. What is the nature of the covalent bond in carbon compounds? |  |
| 2. Why is carbon considered a versatile element? |  |
| 3. What are some common chemical properties of carbon compounds? |  |
| 4. What is the significance of ethanol in carbon compounds? |  |
| 5. How do soaps and detergents differ in their chemical structure and function? |  |






















