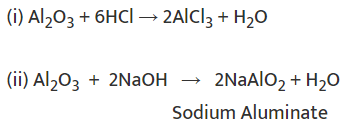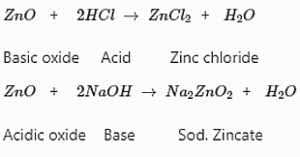- Ionic compounds are formed when a metal loses electrons and a non-metal gains electrons, creating positive and negative ions that attract each other.
- They generally have high melting and boiling points, so option a is false.
- They are usually soluble in polar solvents like water, not in non-polar solvents like kerosene or petrol, so option b is false.
- They conduct electricity only when molten or dissolved in water, not in solid form, so option c is false.
Metals and Non-metals Class 10 Notes Science Chapter 3
| Table of contents |

|
| Introduction |

|
| Physical Properties |

|
| Chemical Properties of Metals |

|
| How do Metals and Non-metals React? |

|
| Ionic Compounds |

|
| Occurrence of Metals |

|
| Corrosion |

|
Introduction
Metals and non-metals are an important part of our daily lives, from the strong materials used in buildings to essential gases like oxygen. Metals like iron and copper are strong and conduct electricity, while non-metals like silicon and carbon are brittle and do not conduct electricity. This chapter explains their special properties, uses, and how they are processed, showing their connection to everyday life.
Physical Properties
Physical properties are characteristics of materials that we can see or measure without changing the material's basic identity. Imagine you have a piece of chocolate. Some of its physical properties are: Color, Texture, Hardness, Melting Point, Taste, Density, Opacity.
(a) Metals
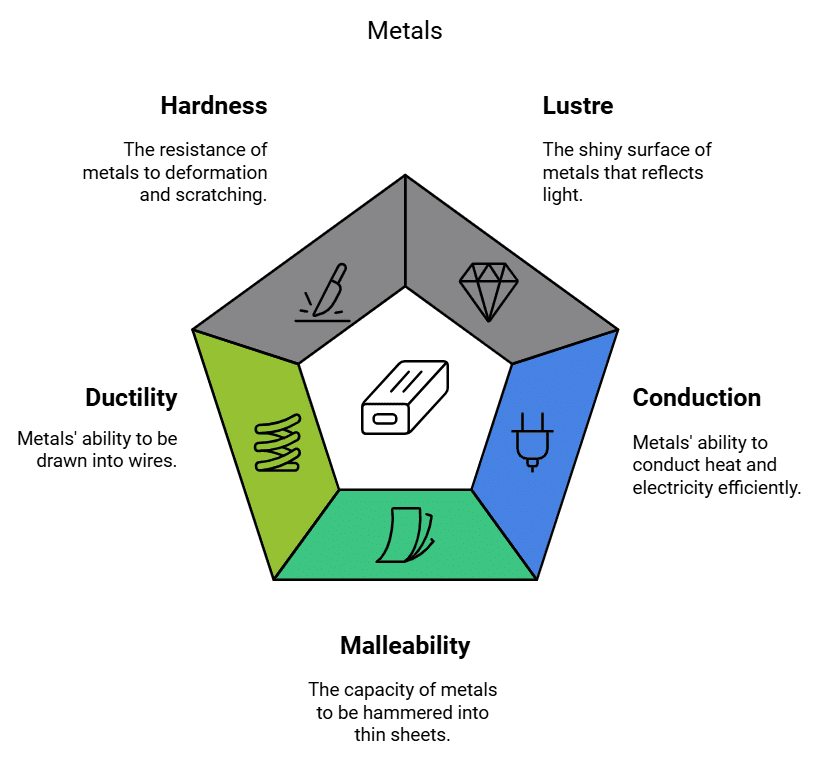 Metals are known for their unique characteristics:
Metals are known for their unique characteristics:
1. Lustre: Metals have a shiny and reflective surface, often described as having a metallic lustre. This property makes them attractive and valuable for various applications, such as jewellery and coins.
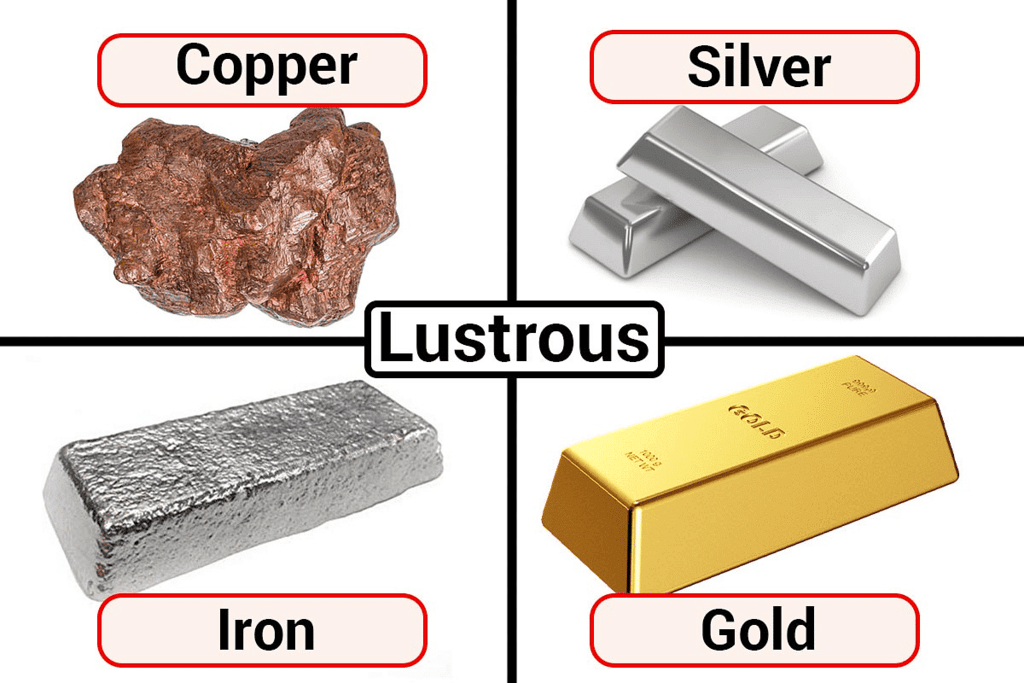 Metals are lustrous
Metals are lustrous
2. Hardness: The hardness of a metal refers to its ability to resist local plastic deformation, fracture, and failure once damage has begun. It also determines how well a material's surface resists wear and abrasion. While most metals are relatively hard, they vary in hardness from metal to metal. For example, iron and copper are hard at room temperature, but sodium is so soft that it can be cut with a knife.
3. Malleability: Metals can be hammered or rolled into thin sheets without breaking. This property is known as malleability and allows metals to be shaped into various forms, like aluminum foils and copper sheets. Gold and silver are the most malleable metals.
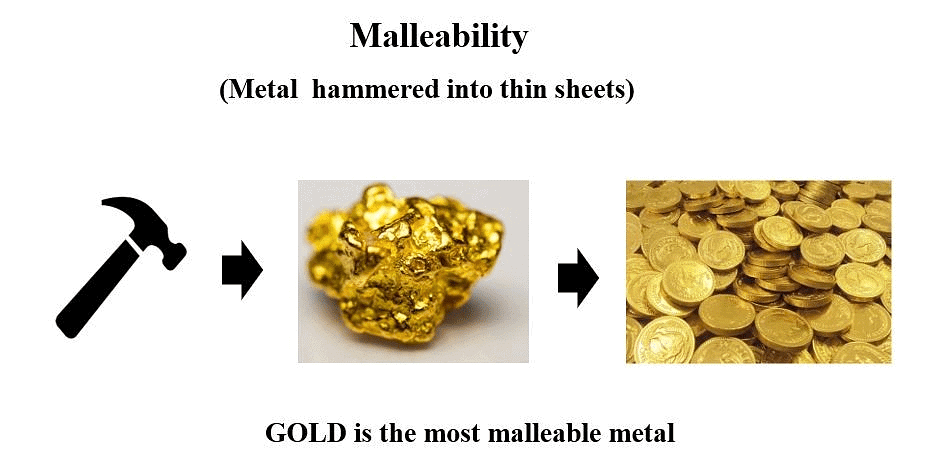 Malleability
Malleability
4. Ductility: Metals can be drawn into thin wires without breaking. This property is called ductility and is crucial for making wires for electrical purposes, such as copper wires in electrical cables. Gold is the most ductile metal (a wire of around 2km length can be drawn from one gram of gold.)
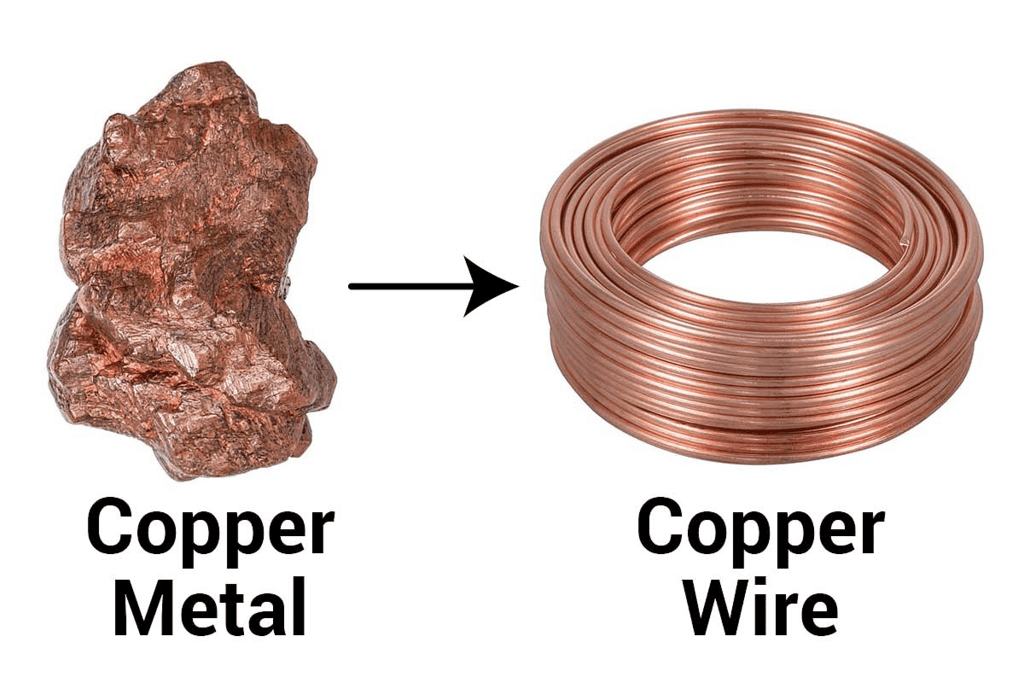 Ductility of Metals
Ductility of Metals
5. Conduction (heat and electricity): Metals are excellent conductors of heat and electricity. They allow heat and electrical currents to flow through them with minimal resistance. This property makes them essential in electrical wiring and cooking utensils. Silver and copper are best conductors of heat. Lead and mercury are comparatively poor conductors of heat.
Do you know why they are good conductors of heat and electricity?
Metals conduct electricity due to presence of free electrons
Due to conductivity of metals, electric wires (which are mostly made of copper, aluminium, steel, silver) are coated with polyvinyl chloride (PVC) to prevent electric shock and other accidents. PVC is an insulator, which means it doesn't conduct electricity, so even if you touch the wire, the current won't pass through you. PVC is also fire resistant and easy to use, and can last for about 40 years.
6. Sonority: Sonority is the property of metals to produce sound when struck against a hard surface. Metals that exhibit sonority are considered sonorous. Most metals are sonorous, and some examples include copper, aluminum, tin, silver, and iron.
 Sonority
Sonority
7. Density: Metals are generally dense materials, meaning they have a relatively high mass for their volume. This property makes them suitable for making heavy machinery and structural components.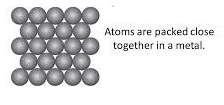
8. Melting and Boiling Points: Metals usually have high melting and boiling points. For example, iron melts at around 1,535 degrees Celsius. The metals have a closely packed structure and have a strong intermolecular force of attraction. To break these intermolecular force of attraction, more heat is required and therefore they have a high melting point except for some metals like gallium and cesium.
(b) Non-Metals
Non-metals, on the other hand, have properties that are quite different from metals:
1. Lack of Lustre: Non-metals do not have the shiny appearance of metals; instead, they can be dull or have various colours.
 Non metals Lack Lustre
Non metals Lack Lustre
3. Brittleness: Many non-metals are brittle and tend to break or crumble when subjected to force. For example, sulfur and phosphorus are brittle non-metals. Non Metals are not Malleable or Ductile.

4. Low Density: Non-metals usually have lower density compared to metals. This means they have a lower mass for a given volume.
5. Low Melting and Boiling Points: Non-metals often have low melting and boiling points. Non-metals are held by weak intermolecular force of attraction, so less heat is required to break them. As a result, they have low melting points.
6. Variety of States: Non-metals can exist in different states at room temperature. For example, oxygen is a gas, while bromine is a liquid, and sulfur is a solid.
Understanding these physical properties is essential as they form the basis for classifying elements as metals or non-metals. These properties also determine the various applications of these elements in our daily lives and various industries.
Below is a summary table containing the key properties of both Metals and Non-Metals for your reference.
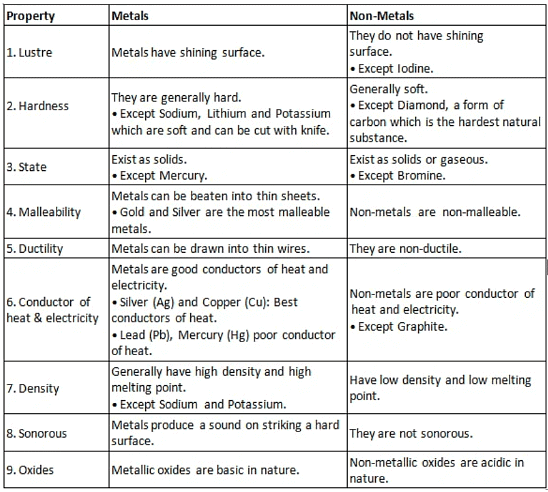
We cannot group elements according to their physical properties alone, as there are many exceptions
- All metals except mercury (liquid) exist as solids at room temperature as metals have high melting points but gallium and caesium have very low melting points. These two metals will melt if you keep them on your palm.
- Iodine is a non-metal, but it is lustrous.
- Carbon is a non-metal that can exist in different forms. Each form is called an allotrope. Diamond, an allotrope of carbon, is the hardest natural substance known and has a very high melting and boiling point. Graphite, another allotrope of carbon, is a conductor of electricity.
- Alkali metals (lithium, sodium, potassium) are so soft that they can be cut with a knife. They have low densities and low melting points. (Alkali metals are a group of chemical elements that are located in the first column of the periodic table, also known as group 1.)
Elements can be more clearly classified as metals and non-metals on the basis of their chemical properties.
Chemical Properties of Metals
(a) Reaction of metals with air
Metals combine with oxygen to form metal oxide.
Metal + O2 → Metal oxide
Examples: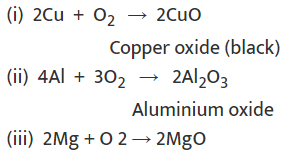
The reactivity of different metals is different with O2.
- Na and K react so vigorously that they catch fire when exposed to air; therefore, they are kept immersed in kerosene.
- Surfaces of Mg, Al, Zn, and Pb are covered with a thin layer of oxide which prevents them from further oxidation.
- Fe does not burn on heating, but iron filings burn vigorously.
- Cu does not burn under normal conditions, but can form copper oxide when heated.
- Au and Ag do not react with oxygen.
Anodising is a process of forming a thick oxide layer of aluminium. Aluminium develops a thin oxide layer when exposed to air. This aluminium oxide coat makes it resistant to further corrosion. The resistance can be improved further by making the oxide layer thicker. During anodising, a clean aluminium article is made the anode and is electrolysed with dilute sulphuric acid. The oxygen gas evolved at the anode reacts with aluminium to make a thicker protective oxide layer. This oxide layer can be dyed easily to give aluminium articles an attractive finish.
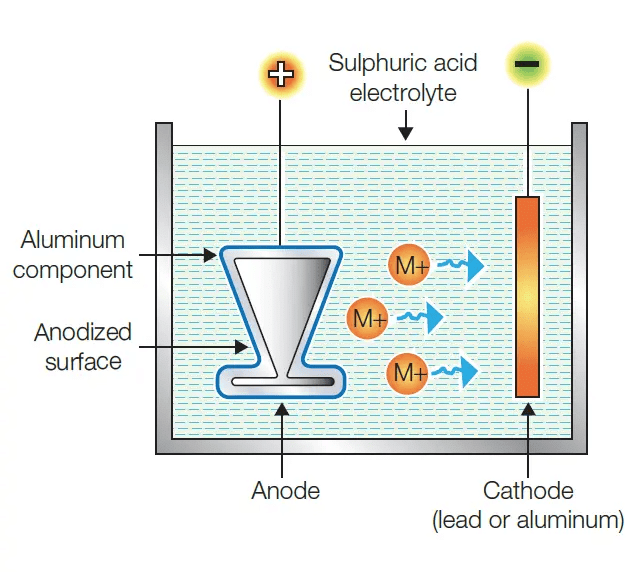 Anodising Process
Anodising Process
Metal oxides are basic in nature. But some metal oxides, such as aluminium oxide, zinc oxide, etc., show both acidic as well as basic behaviour. Such metal oxides which react with both acids as well as bases to produce salts and water are known as amphoteric oxides.
Examples:
(b) Reaction of metals with water
Metals react with water and produce a metal oxide and hydrogen gas. Metal oxides that are soluble in water dissolve in it to further form metal hydroxide. However all metals do not react with water.
- Metal + Water → Metal oxide + Hydrogen
- Metal oxide + Water → Metal hydroxide
Examples:
(a) Metals like potassium and sodium react violently with cold water. In case of sodium and potassium, the reaction is so violent and exothermic that the evolved hydrogen immediately catches fire.
- 2Na(s) + 2H2 O(l) → 2NaOH(aq) + H2 (g) + heat energy
- 2K(s) + 2H2O(l) → 2KOH(aq) + H2(g) + heat energy
(b) Calcium starts floating because the bubbles of hydrogen gas formed stick to the surface of the metal.
- Ca(s) + 2H2 O(l) → Ca(OH)2(aq) + H2(g)
(c) Magnesium does not react with cold water. It reacts with hot water to form magnesium hydroxide and hydrogen. It also starts floating due to the bubbles of hydrogen gas sticking to its surface.
- Mg(s) + 2H2O(l) → Mg(OH)2 (aq) + H2(g)
(d) Metals like aluminium, iron and zinc do not react either with cold or hot water. However, they react with steam to form metal oxides and hydrogen.
- 2Al(s) + 3H2O(g) → Al 2O3(s) + 3H2(g)
- 3Fe(s) + 4H2O(g) → Fe3O4(s) + 4H2(g)
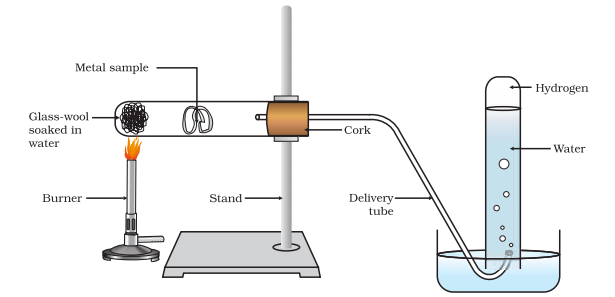 Action of steam on a metal
Action of steam on a metal
Metals such as lead, copper, silver and gold do not react with water at all.
(c) Reaction of metals with acids (Dilute)
Metal + Dilute acid → Salt + H2
Note: Copper (Cu), Silver (Ag), and Mercury (Hg) do not react with dilute acids
Examples
(i) Fe + 2HCl → FeCl2 + H2
(ii) Mg + 2HCl → MgCl2+ H2
(iii) Zn + 2HCl → ZnCl2 + H2
(iv) 2Al + 6HCl → 2AlCl3 + 3H2
Exception:- Hydrogen gas is not evolved when a metal reacts with nitric acid. It is because HNO3 is a strong oxidising agent. It oxidises the H2 produced to water and itself gets reduced to any of the nitrogen oxides (N2O, NO, NO2). But magnesium (Mg) and manganese (Mn) react with very dilute HNO3 to evolve H2 gas.
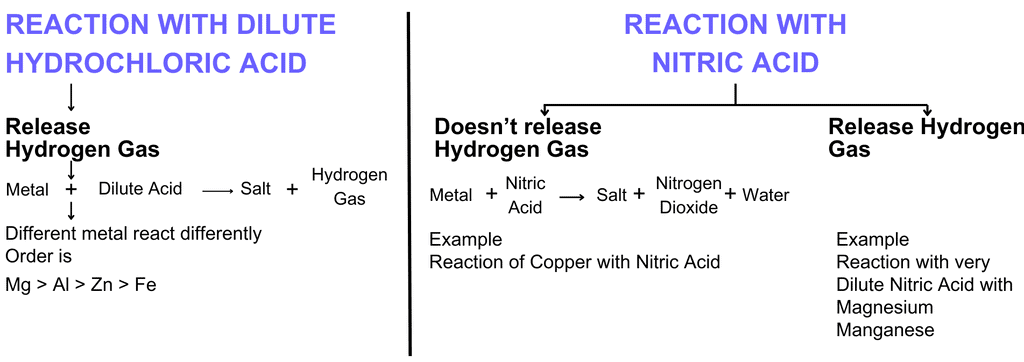 And Cu doesn't react with HCl.
And Cu doesn't react with HCl.
Aqua Regia, (Latin for ‘royal water’) is a freshly prepared mixture of concentrated hydrochloric acid and concentrated nitric acid in the ratio of 3:1. It can dissolve gold, even though neither of these acids can do so alone. Aqua Regia is a highly corrosive, fuming liquid. It is one of the few reagents that is able to dissolve gold and platinum
(d) Reaction of Metals with Solutions of other Metal Salts
Metal A + Salt solution B → Salt solution A + Metal B
Reactive metals can displace less reactive metals from their compounds in solution form.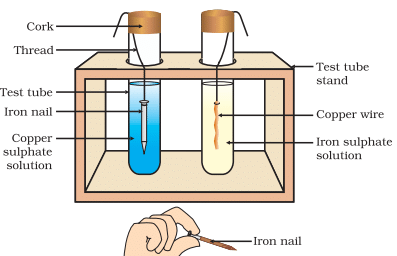 In test tube 1 : Fe + CuSO4 → FeSO4 + Cu
In test tube 1 : Fe + CuSO4 → FeSO4 + Cu
- Here, Fe displaces Cu from its salt solution as Fe is more reactive than Cu.
In test tube 2: Cu + FeSO4 → No reaction
- Cu cannot displace Fe from its salt solution as Cu is less reactive than Fe.
(e) The Reactivity Series
The reactivity series is a list of metals arranged in the order of their decreasing activities.
Mnemonic to remember:- Popular Scientist Can Make A Zoo In Low Humid Climate More Accurately.
Po:- Potassium(K), S:- Sodium(Na), Ca:- Calcium(Ca), A:- Aluminium (Al), Z:- Zinc (Zn), I:- Iron(Fe), L:- Lead(Pb), H:- Hydrogen (H), C:- Copper(Cu), M:- Mercury (Hg), A:-Silver(Ag)
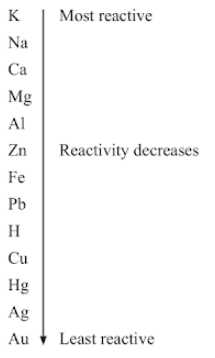 Reactivity Series
Reactivity Series
How do Metals and Non-metals React?
Reactivity of elements is the tendency to attain a filled valence shell. Atoms of the metals lose electrons from their valence shell to form cations. Atoms of non-metals gain electrons in the valence shell to form an anion.
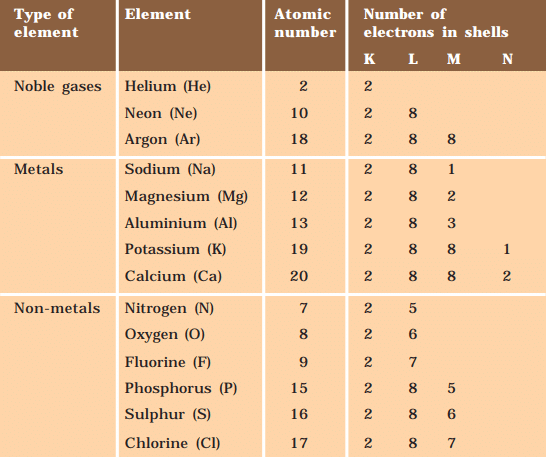
Example: Formation of NaCl
Sodium(Na)
- A sodium atom has one electron in its outer shell.
- When it loses this electron, its next shell (the L shell) becomes the outermost shell and has a full set of eight electrons, making it stable.
- Since it now has 10 electrons but still 11 protons, it becomes positively charged and is called a sodium ion (Na⁺).
Chlorine(Cl)
- on the other hand, has seven electrons in its outer shell and needs one more to complete its set of eight electrons.
- When sodium and chlorine react, the sodium atom gives away its extra electron to chlorine.
- After receiving the electron from sodium, chlorine, which originally had 17 electrons, now has 18 electrons but still has 17 protons, making it negatively charged.
- This results in a chloride ion (Cl⁻). So, sodium and chlorine work together by sodium giving away an electron and chlorine accepting it.
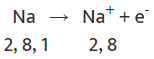
Sodium cation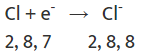
Chloride anion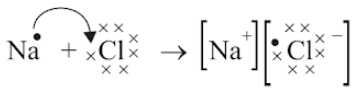
Sodium and chloride ions, being oppositely charged, attract each other and are held by strong electrostatic forces of attraction to exist as sodium chloride (NaCl). It should be noted that sodium chloride does not exist as molecules but aggregates of oppositely charged ions.
Ionic Compounds
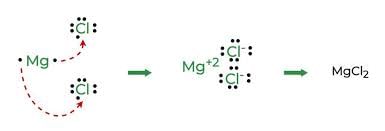
The compounds formed by the transfer of electrons from a metal to a non-metal are called ionic compounds or electrovalent compounds.
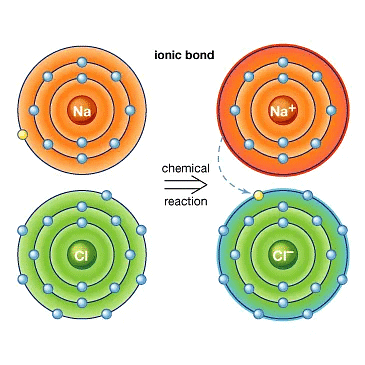 Formation of Ionic Compounds
Formation of Ionic Compounds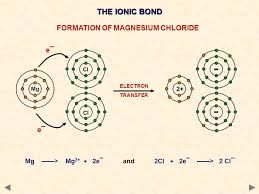
Properties of Ionic Compounds
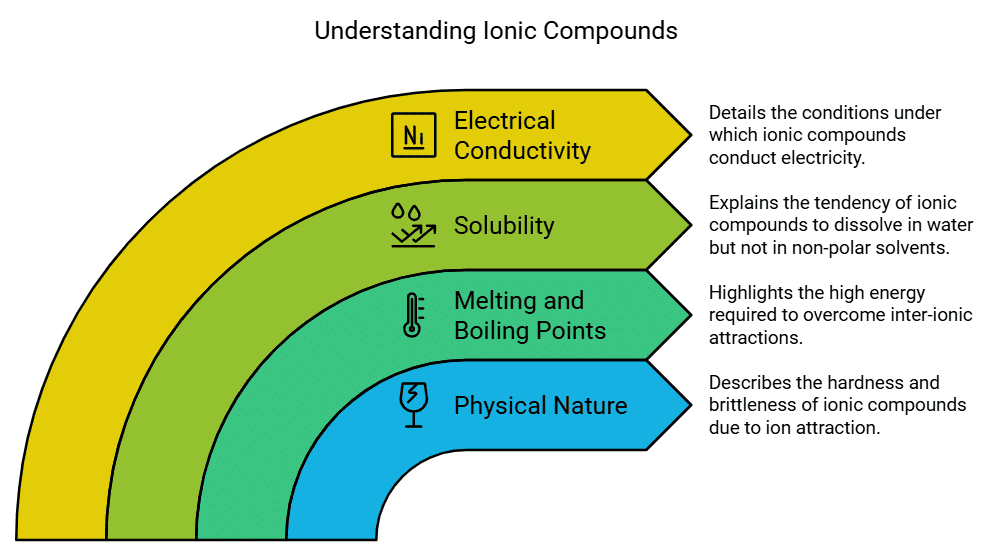
- Physical nature: Ionic compounds are solid substances and possess a certain level of hardness due to the powerful attraction between the positively and negatively charged ions. Typically, these compounds exhibit brittleness and tend to fracture into fragments when subjected to pressure.
- Melting and Boiling Point: Ionic compounds exhibit high melting and boiling points due to the presence of strong inter-ionic attractions, which require a significant amount of energy to be overcome.
- Solubility: Electrovalent compounds usually dissolve in water but do not dissolve in solvents like kerosene or petrol.
- Conduction of electricity: When an ionic compound is dissolved in water, the resulting solution contains ions that can migrate towards the electrodes when an electric current is applied. In contrast, solid ionic compounds do not conduct electricity because the ions are unable to move due to their fixed arrangement. However, when ionic compounds are in the molten state, they can conduct electricity because the heat overcomes the electrostatic forces holding the oppositely charged ions together. As a result, the ions become mobile and can freely move, facilitating the conduction of electricity.
Occurrence of Metals
- Minerals: The elements or compounds which occur naturally in the earth’s crust are called minerals.
- Ores: Minerals that contain a very high percentage of particular metal and the metal can be profitably extracted from it, such minerals are called ores.
Extraction of metals from their ores
Step 1. Enrichment of ores.
Step 2. Extraction of metals.
Step 3. Refining of metals.
Enrichment of Ores
Enrichment of ores refers to the process of eliminating impurities, such as soil, sand, stone, and silicates, from mined ores. Ores commonly contain a variety of unwanted substances called gangue. The primary objective of concentration is to separate these impurities from the ores. Concentration methods rely on the physical and chemical properties of the ores. Examples of such processes include gravity separation, electromagnetic separation, and froth flotation.
Extracting Metals Low in Activity Series
During self-reduction, when sulphide ores of metals such as Hg, Pb, Cu, etc., are heated in the presence of air, a portion of the ore is converted into an oxide. This oxide then reacts with the remaining sulphide ore to produce crude metal and sulphur dioxide. Notably, no external reducing agent is employed in this process.
- 2HgS(Cinnabar)+3O2(g)+heat→2HgO(crude metal)+2SO2(g)
2HgO(s)+heat→2Hg(l)+O2(g) - Cu2S(Copper pyrite)+3O2(g)+heat→2Cu2O(s)+2SO2(g)
2Cu2O(s)+Cu2S(s)+heat→6Cu(crude metal)+SO2(g) - 2PbS(Galena)+3O2(g)+heat→2PbO(s)+2SO2(g)
PbS(s)+2PbO(s)→2Pb(crude metal)+SO2(g)
Extracting Metals in the Middle of Activity Series
Metals in the middle of the activity series, such as iron, zinc, lead, and copper, have a moderate level of reactivity. These metals are commonly found in nature as sulphides or carbonates. Obtaining these metals from their oxides is easier compared to extracting them from sulphides and carbonates.
Therefore, before the reduction process, sulphide ores and carbonate ores need to be converted into metal oxides.
1. Conversion of Ores to Oxides
(a) Roasting of Sulphide Ores
- Sulphide ores are transformed into oxides through intense heating in the presence of an excess of air, a process called roasting.
- Example: 2ZnS (s) + 3O2 (g) → 2ZnO(s) + 2SO2 (g)
(b) Calcination of Carbonate Ores
- Carbonate ores are converted into oxides by strong heating in a limited air environment, known as calcination.
- Example: ZnCO3 (s)
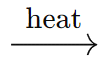 ZnO(s) + CO2 (g)
ZnO(s) + CO2 (g)
2. Reduction of Metal Oxides
- The metal oxides are then reduced to the corresponding metals by using suitable reducing agents such as carbon.
- For example, when zinc oxide is heated with carbon, it is reduced to metallic zinc.
ZnO(s) + C(s) → Zn(s) + CO(g) - Displacement reactions can also be used. Highly reactive metals such as sodium, calcium, or aluminium can displace less reactive metals from their compounds.
Example: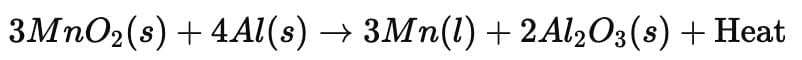
3. Thermite Reaction
- These displacement reactions are highly exothermic. The amount of heat evolved is so large that the metals are produced in the molten state.
- The reaction of iron(III) oxide (Fe₂O₃) with aluminium is used in practical applications such as joining railway tracks or repairing cracked machine parts. This is called the thermite reaction.
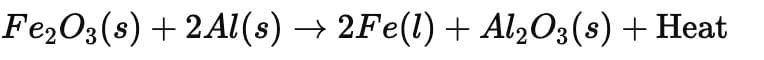
Extracting Metals towards the Top of the Activity Series
Metals that are high up in the reactivity series are extremely reactive. They cannot be extracted from their compounds through the process of heating with carbon. For instance, carbon is unable to reduce the oxides of metals like sodium, magnesium, calcium, and aluminum to obtain the pure metals.
- The reason behind this is that these metals have a stronger attraction to oxygen than carbon does.
- Therefore, these metals are obtained through electrolytic reduction, where the process involves the electrolysis of their molten chlorides.
- During this process, the metals are deposited at the cathode (the negatively charged electrode), while chlorine gas is liberated at the anode (the positively charged electrode).
- At the cathode (reduction):
Na+(molten)+e−→Na(s)
Metal is deposited - At the anode (oxidation):
2Cl−(molten)→Cl2(g)+2e–
Chlorine gas is liberated.
Important terms
(a) Gangue: Ores are usually contaminated with large amounts of impurities such as soil, sand etc. called gangue.
(b) Roasting: The sulphide ores are converted into oxides by heating strongly in the presence of excess air. This process is called roasting.
2ZnS + 3O2 →(Heat) 2ZnO + 2SO2
(c) Calcination: The carbonate ores are changed into oxides by heating strongly in limited air. This process is called calcination.
ZnCO3 →(Heat) ZnO + CO2
(d) Reduction: Metal oxides are reduced to corresponding metals by using a reducing agent like carbon.
ZnO + C → Zn + CO
Refining of Metals
The most widely used method for refining impure metal is electrolytic refining.
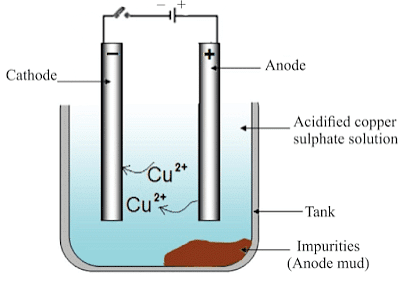 Electrolytic Refining
Electrolytic Refining
(i) Anode: Impure copper
(ii) Cathode: Strip of pure copper
(iii) Electrolyte: Solution of acidified copper sulphate
- On passing the current through the electrolyte, the impure metal from the anode dissolves into the electrolyte.
- An equivalent amount of pure metal from the electrolyte is deposited at the cathode.
The insoluble impurities settle down at the bottom of the anode and are called anode mud.
Corrosion
The surface of some metals gets corroded when they are exposed to moist air for a long period. This is called corrosion.
 CorrosionExamples:
CorrosionExamples:
(i) Silver becomes black when exposed to air as it reacts with air to form a coating of silver sulphide.
(ii) Copper reacts with moist carbon dioxide in the air and gains a green coat of copper carbonate.
(iii) Iron when exposed to moist air acquires a coating of a brown flaky substance called rust.
Prevention of Corrosion
- The rusting of iron can be prevented by painting, oiling, greasing, galvanizing, chrome plating, anodizing or making alloys.
- Galvanization: It is a method of protecting steel and iron from rusting by coating them with a thin layer of zinc.
- Alloying: Alloying is a useful technique for enhancing the properties of metals. By mixing a metal with other substances, we can achieve the desired characteristics.
For example, iron is commonly used but not in its pure form because pure iron is too soft and stretches easily when heated. However, when iron is combined with a small amount of carbon (about 0.05%), it becomes much harder and stronger. Additionally, mixing iron with nickel and chromium produces stainless steel, which is both hard and resistant to rust.
Essentially, alloying changes the properties of the base metal. An alloy is a uniform mixture of two or more metals, or a metal and a non-metal. To make an alloy, the primary metal is melted, and other elements are added in specific proportions. The mixture is then cooled to solidify the alloy.
 Examples of alloy:
Examples of alloy:
(i) Iron: Mixed with a small amount of carbon becomes hard and strong.
(ii) Steel: Iron + Nickel and chromium
(iii) Brass: Copper + Zinc
(iv) Bronze : Copper + Tin (Sn)
(v) Solder: Lead + tin
(vi) Amalgam: If one of the metals is mercury (Hg)
Alloys generally have lower electrical conductivity and melting points compared to pure metals. For instance, brass, which is an alloy of copper and zinc (Cu and Zn), and bronze, an alloy of copper and tin (Cu and Sn), are not as good at conducting electricity as pure copper, which is commonly used in electrical circuits. Similarly, solder, an alloy of lead and tin (Pb and Sn), has a low melting point and is used for joining electrical wires.
Pure gold, known as 24 carat gold, is very malleable and soft. It is, therefore, not suitable for making jewellery. It is alloyed with either silver or copper to make it hard. Generally, in India, 22 carat gold is used for making ornaments. It means that 22 parts of pure gold is alloyed with 2 parts of either copper or silver
Frequently Asked Questions (FAQs)
Q1. What are the physical properties of metals?
Ans. Metals are typically hard, dense, shiny, malleable, ductile, and good conductors of heat and electricity. They have a high boiling and melting point and are usually solid at room temperature. $$$
Q2. What are the physical properties of non-metals?
Ans. Non-metals are usually dull, brittle, and poor conductors of heat and electricity. They have low boiling and melting points and can exist in all three states of matter at room temperature. Non-metals are not malleable or ductile.
Q3. What is the difference between metals and non-metals?
Ans. The primary difference between metals and non-metals is their physical properties. Metals are usually hard, dense, and good conductors of heat and electricity, while non-metals are brittle, dull, and poor conductors of heat and electricity. Additionally, metals tend to form cations, while non-metals form anions.
Q4. What are some common uses of metals?
Ans. Metals have a variety of uses, including construction, transportation, electrical wiring, and the production of tools and appliances. Some metals, such as gold and silver, are used in jewelry and currency. Iron and steel are commonly used in construction and manufacturing.
Q5. What are some common uses of non-metals?
Ans. Non-metals are used in a variety of applications, such as the production of plastics, ceramics, and semiconductors. Non-metals such as oxygen and nitrogen are essential for life and are present in the atmosphere. Non-metals such as sulfur and chlorine are used in the production of a variety of chemicals.
|
80 videos|569 docs|80 tests
|
FAQs on Metals and Non-metals Class 10 Notes Science Chapter 3
| 1. What are the main physical properties of metals? |  |
| 2. How do metals and non-metals react chemically? |  |
| 3. What are ionic compounds and how are they formed? |  |
| 4. Where are metals commonly found in nature? |  |
| 5. What is corrosion and how can it be prevented? |  |