NEET Exam > NEET Notes > Chemistry Class 12 > Mind Map: p-Block Elements
Mind Map: p-Block Elements | Chemistry Class 12 - NEET PDF Download
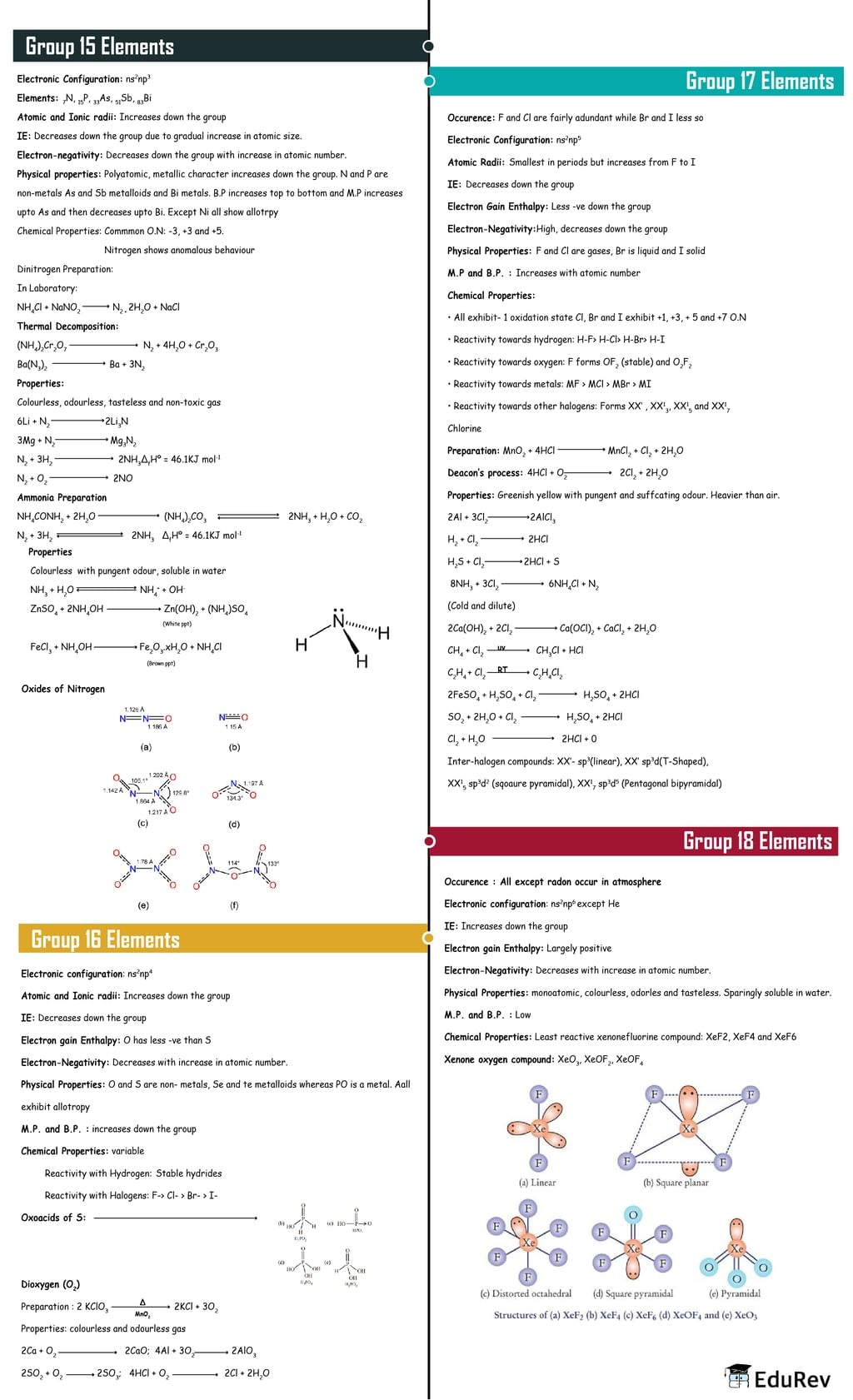
The document Mind Map: p-Block Elements | Chemistry Class 12 - NEET is a part of the NEET Course Chemistry Class 12.
All you need of NEET at this link: NEET
|
75 videos|278 docs|78 tests
|
FAQs on Mind Map: p-Block Elements - Chemistry Class 12 - NEET
| 1. What are p-block elements? |  |
Ans. p-block elements are the elements in the periodic table that belong to groups 13 to 18. They are named so because their valence electrons occupy the p orbital. These elements exhibit a wide range of chemical properties and can be found in various compounds.
| 2. What are the general characteristics of p-block elements? |  |
Ans. The general characteristics of p-block elements include having valence electrons in the p orbital, variable oxidation states, the ability to form covalent bonds, and the tendency to form compounds with nonmetals. They also exhibit diverse physical and chemical properties due to the presence of different elements within this block.
| 3. How do p-block elements contribute to the periodic table? |  |
Ans. p-block elements occupy the rightmost side of the periodic table, contributing to its overall structure and properties. These elements play a crucial role in the formation of various compounds, including organic compounds, and exhibit a wide range of chemical reactivity. They also contribute to the diversity of properties and behaviors observed across different elements.
| 4. What are some important applications of p-block elements? |  |
Ans. p-block elements find numerous applications in various fields. For example, boron and silicon are used in semiconductor devices, nitrogen is crucial for the production of fertilizers, and carbon is the key element in organic chemistry. Additionally, elements like sulfur find applications in the production of sulfuric acid, while noble gases are used in lighting and cryogenic applications.
| 5. How do the properties of p-block elements change across a period? |  |
Ans. The properties of p-block elements change systematically across a period. As you move from left to right, the atomic size generally decreases, electronegativity increases, and the tendency to gain electrons increases. The metallic character decreases, and nonmetallic character increases. The reactivity of elements also varies, with some elements being highly reactive (e.g., alkali metals) and others being relatively inert (e.g., noble gases).
Related Searches






















