Index: Chemical Reactions & Equations | Science Class 10 PDF Download
| Table of contents |

|
| 1. Topicwise division of the Chapter |

|
| 2. NCERT |

|
| 3. Questions from Famous Books |

|
| 4. Practice |

|
| 5. Summary |

|
Have you ever witnessed a paper burning? An entirely white piece of paper turns into ashes after being completely burnt. This process is nothing but a type of chemical reaction only.
In this EduRev index document, you will get everything to understand the chemical reactions. It includes all of the information needed to comprehend the chapter as well as enough of practise material to help you study for the examinations.
In this chapter EduRev provides you with:
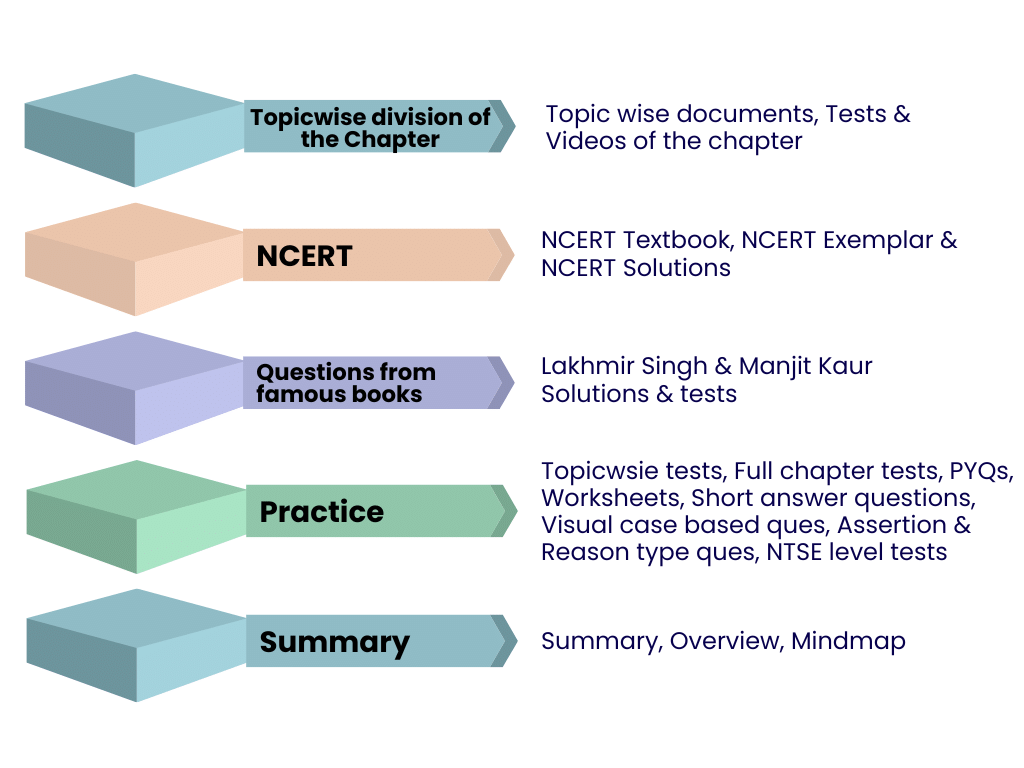
1. Topicwise division of the Chapter
You will get the notes, tests, and videos for each topic in the chapter in the Topicwise section. You can understand each and every topic in detail with the help of documents and videos. After reading the document and watching the videos for all the topics, you can revise it and start attempting tests to strengthen your practice.
The flowchart below is showing all the topics in the chapter Chemical Reactions and Equations:
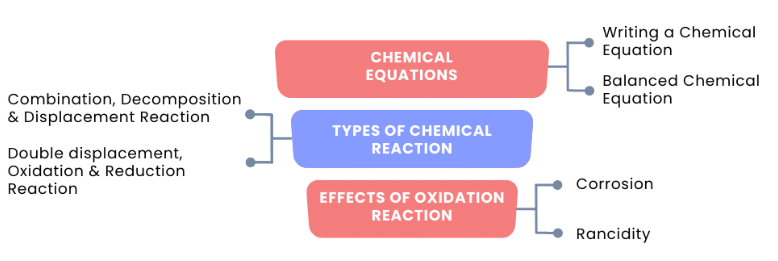
(a) Chemical Equations
(b) Types of Chemical Reactions
- Video: Introduction to Chemical Reactions
- Video: Types of Chemical Reactions
- Doc: Chemical Reactions: Types, Corrosion & Rancidity
- Video: Rancidity
Video: Corrosion
- Video: What triggers a chemical reaction?
- Test: Types Of Displacement Reactions
- Doc: Oxidation and Reduction
- Test: Redox Reactions
(c) Effects of Oxidation Reaction
2. NCERT
The NCERT textbooks are the most important for students to study in order to comprehend the concepts; since most questions only come from NCERT books, it is crucial for them to get a fundamental grasp of the chapter. EduRev offers NCERT textbooks for every chapter, answers to every NCERT question, and NCERT Exemplars as a whole. To read the NCERT content, click the links below.
- Doc: NCERT Textbook: Chemical Reactions and Equations
- Doc: NCERT Solutions: Chemical Reactions and Equations
- Doc: NCERT Exemplar: Chemical Reactions & Equations
3. Questions from Famous Books
Lakhmir and Manjit Kaur's book is one of the helpful resources for students to read the theory and practise questions from it as it provides questions in abundance and simplifies theory. EduRev provides you with questions (with solutions) from not only the NCERT textbook but also from some famous books like Lakhmir Singh & Manjit Kaur from which questions are asked in the exams.
- Doc: Lakhmir Singh & Manjit Kaur: Chemical Reactions and Equations, Solutions- 1
- Doc: Lakhmir Singh & Manjit Kaur: Chemical Reactions and Equations, Solutions- 2
- Doc: Lakhmir Singh & Manjit Kaur: Chemical Reactions and Equations, Solutions- 3
- Test: Lakhmir Singh & Manjit Kaur Test: Chemical Reactions and Equations
4. Practice
After fully understanding all the topics and concepts, it's time to put your knowledge to use by attempting tests and working through questionnaires in documents. EduRev offers you well-examined tests that are tailored to your examinations and papers with clear explanations for your benefit. All tests are multiple-choice questions, which can help you prepare for a real exam.
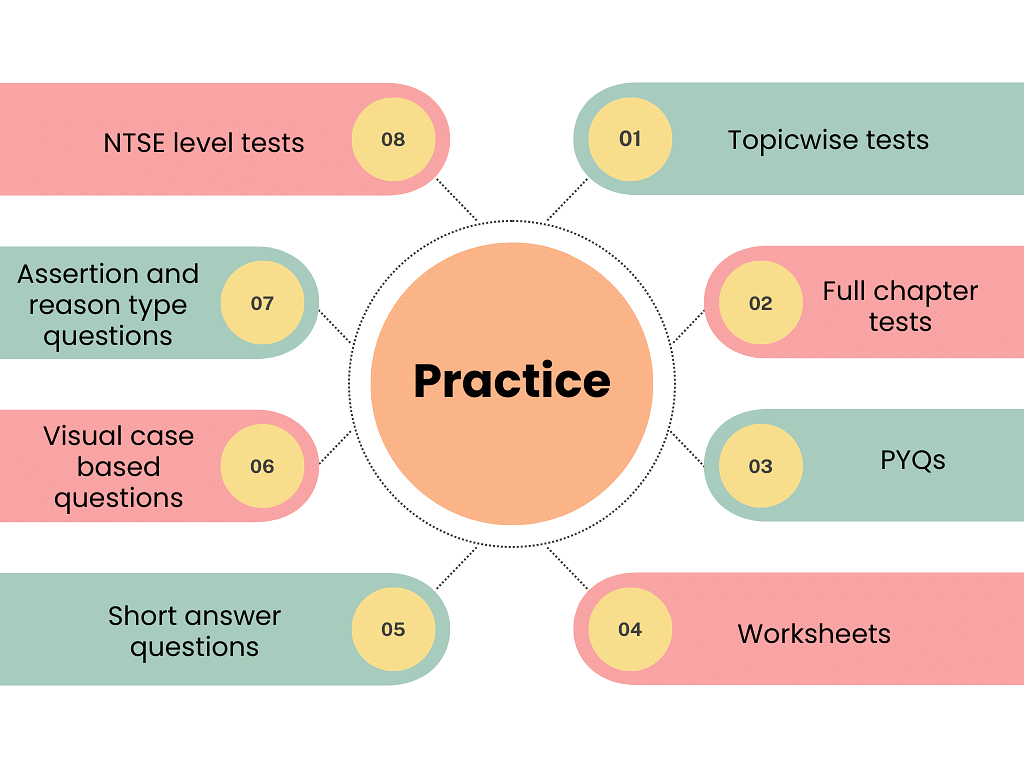
(a) Topicwise Tests
Test: Redox Reactions
(b) Full Chapter Tests & Documents
- Test: Chemical Reactions and Equations
- Test: Chemical Reactions And Equations (Easy)
- Test: Chemical Reactions And Equations (Medium)
- Test: Chemical Reactions And Equations (Hard)
- Doc: Practice Questions: Chemical Reactions and Equations
- Test: Important Questions (1 mark): Chemical Reactions And Equations
- Test: Important Questions (2 marks): Chemical Reactions And Equations
(c) PYQs
(d) Worksheets
- Doc: Worksheet (1): Chemical Reactions and Equations
- Doc: Worksheet (2): Chemical Reactions and Equations
(e) Short Answer Questions
(f) Visual Case-based Questions
- Doc: Chemical Reactions & Equations: Visual Case Based Type Questions- 1
- Doc: Chemical Reactions & Equations: Visual Case Based Type Questions- 2
(g) Assertions & Reason type Questions
- Doc: Chemical Reactions & Equations: Assertions & Reason Type Questions- 1
- Doc: Chemical Reactions & Equations: Assertions & Reason Type Questions- 2
(e) NTSE Level Tests
- NTSE Level Test: NTSE Level Test: Chemical Reactions and Equations
5. Summary
Even after practising and grasping the concepts, you still need to go over the topics repeatedly and review the concepts. To help you with this, EduRev offers chapter summaries that include overview documents, summary documents, and mind maps, which are the most effective tools for reviewing the concepts.
|
85 videos|437 docs|75 tests
|
FAQs on Index: Chemical Reactions & Equations - Science Class 10
| 1. What is the topicwise division of the chapter? |  |
| 2. What is NCERT? |  |
| 3. Can you provide some questions from famous books related to chemical reactions and equations? |  |
| 4. How can one practice chemical reactions and equations? |  |
| 5. Can you provide a summary of the chapter on chemical reactions and equations? |  |

|
Explore Courses for Class 10 exam
|

|