How to Master Classification of Elements & Periodicity in Properties for NEET? | Chemistry Class 11 PDF Download
Let's explore the Class 11 Chemistry chapter called "Classification of Elements & Periodicity in Properties" and understand how it's crucial for the NEET exam. By studying the past years' NEET Previous Year Questions (2016-2025), we can see that this chapter is highly important in the exam. If you want to score well in NEET, you need to grasp the ideas in this chapter.
Meeting Your Timetable Goals with EduRev!
The study plan for this chapter offers you a schedule to manage your time effectively for learning and practicing the chapter thoroughly. By following this plan diligently, you'll be well-prepared to tackle even the most challenging questions asked in NEET related to each chapter. EduRev makes your preparation easier and saves you time by providing comprehensive resources for each topic. These resources include chapter notes, videos, and tests for every topic and chapter.
To access these valuable resources, including documents, videos, and tests, simply click here.
Topics Covered in the Chapter
The Making of Periodic Table: Understand the historical development of the periodic table and the contributions of various scientists.
Modern Periodic Law & Modern Period Table: Explore the modern periodic law and how it led to the current arrangement of elements in the periodic table.
Nomenclature of Elements with Atomic Number > 100: Learn how elements beyond atomic number 100 are named and categorized.
Electronic Configuration of Elements & Periodic Table: Dive into the electronic configurations of elements and how they relate to their position in the periodic table.
Electronic Configurations & Types of Elements: s, p, d & f-block Elements: Explore the characteristics of s, p, d, and f-block elements based on their electronic configurations.
Periodic Trends in Properties of Elements: Understand the periodic trends in atomic and physical properties of elements as you move across and down the periodic table.
Metals, Non-Metals & Metalloids: Differentiate between metals, non-metals, and metalloids based on their properties and positions in the periodic table.
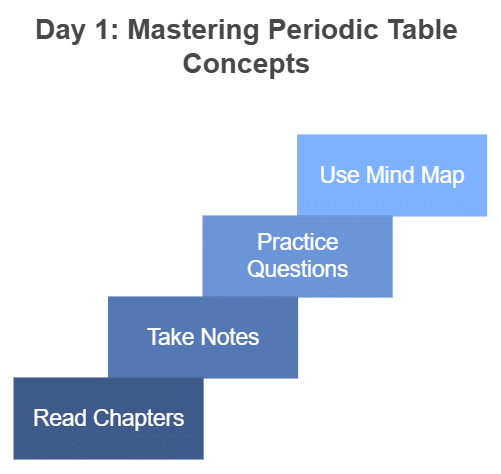
Day 1: The Making of Periodic Table & Modern Periodic Law
- Start by reading the corresponding chapters in your NCERT Chemistry Class 11 textbook.
- Take notes on the key concepts, historical developments, and important scientists.
- After reading, practice questions from the NCERT textbook related to these topics.
- Supplement your understanding with the mind map provided here.
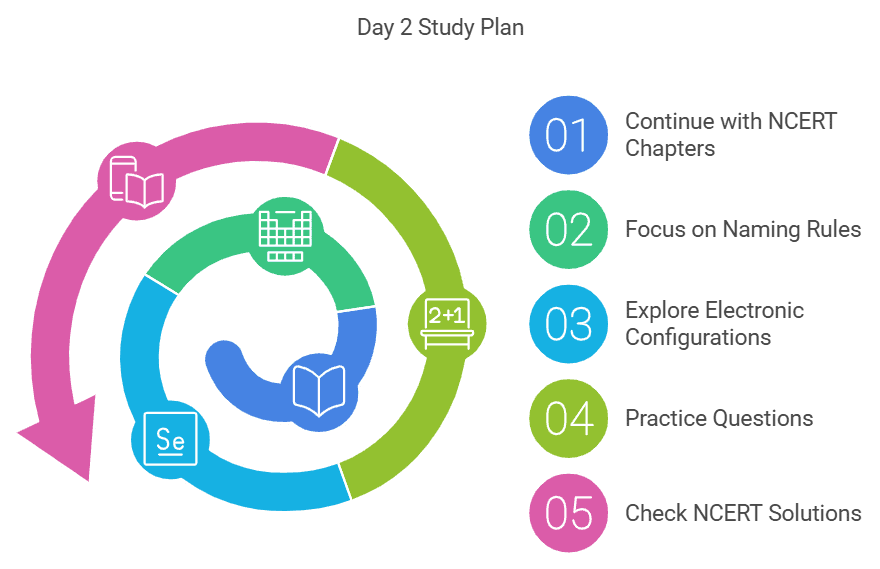
Day 2: Nomenclature of Elements & Electronic Configuration
- Continue with the next chapters in your NCERT textbook.
- Focus on the rules and principles governing the naming of elements with atomic numbers greater than 100.
- Dive into electronic configurations and how they relate to the periodic table.
- Practice questions from the NCERT textbook to reinforce your knowledge.
- Check the NCERT solutions here for any doubts.
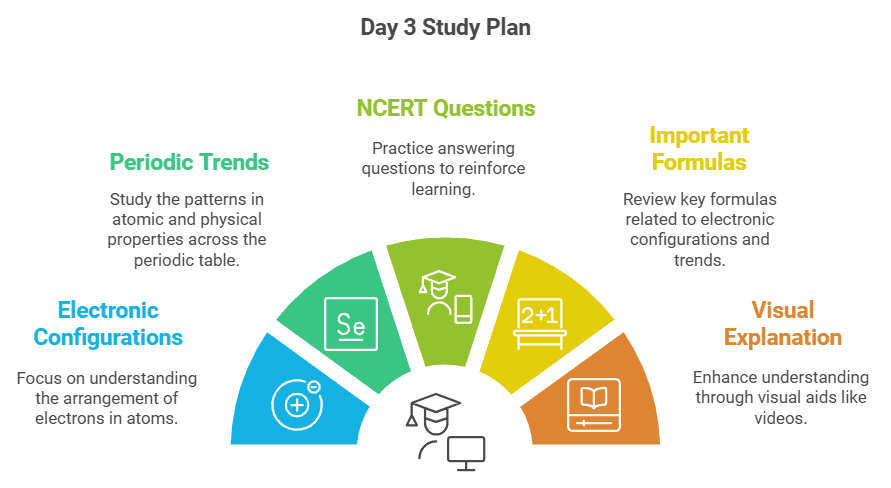
Day 3: Electronic Configurations & Periodic Trends
- Cover the chapters related to electronic configurations and periodic trends.
- Pay special attention to the trends in atomic and physical properties of elements.
- Solve short and long answer questions from the NCERT textbook to consolidate your learning.
- Review the important formulas for this chapter.
- Watch the video here for a visual explanation.
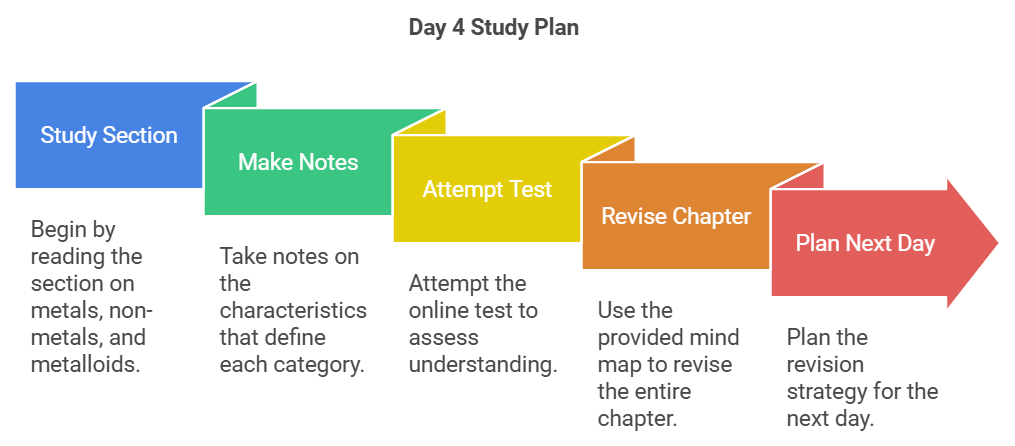
Day 4: Metals, Non-Metals & Metalloids
- Conclude your reading by studying the section on metals, non-metals, and metalloids.
- Make notes on the characteristics that define each category.
- Attempt the test here to assess your understanding.
- Revise the entire chapter using the provided mind map.
- Plan your revision strategy for the next day.
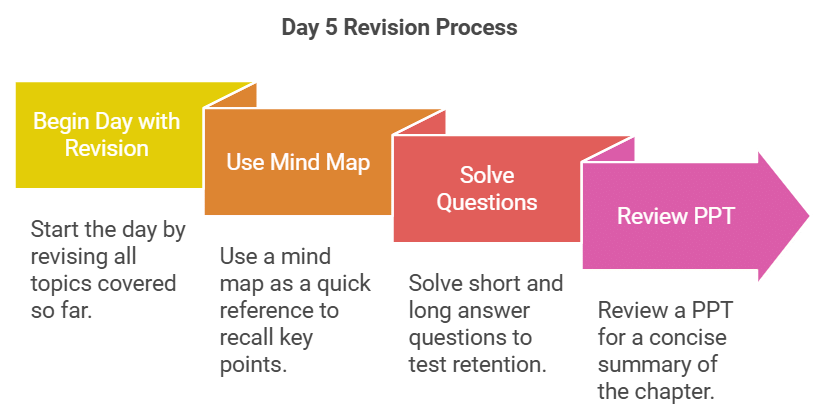
Day 5: Revision
- Begin your day by revising all the topics you've covered so far.
- Use the mind map as a quick reference to recall key points.
- Solve the short and long answer questions here to test your retention.
- Review the PPT here for a concise summary of the chapter.
Remember to read from your NCERT textbook, attempt questions from it, and take help from EduRev for NCERT-based notes and solutions to NCERT questions. EduRev's comprehensive resources, including short and long answer questions, mind maps, and tests, will aid in your thorough preparation for the NEET exam. Stay focused and consistent, and you'll master this important chapter for your NEET journey.
Tips and Tricks
To remember the periodic table's historical development, create an acronym like "SPARK" (Scientists' Periodic Arrangement of Reactions and Knowledge), which will help you recall the contributions of scientists like Mendeleev, Moseley, and others.
Electronic Configuration Mnemonics:
Use "Silly People Don’t Fear Playing" to remember the order of s, p, d, f-block elements and their configurations.
Periodic Trends:
To remember trends in atomic size, ionization energy, and electron affinity, use the phrase: "Aunt Izzy’s Experiments Are Rare" (Aunt = Atomic size, Izzy = Ionization energy, Experiments = Electron affinity, Rare = increases across period, decreases down group).
Visualization:
Make use of EduRev’s mind map to get a clear visual understanding of the periodic table and its trends.
Watch the video on periodic trends to get a deeper grasp of the concepts and how they relate to each other.
Now, let's dive into your study plan and ace the "Classification of Elements & Periodicity in Properties" chapter!
Here are all the important links and topic links for the "Classification of Elements & Periodicity in Properties" chapter:
Important Links:
Topic Links:
- The Making of Periodic Table
- Modern Periodic Law & Modern Periodic Table
- Nomenclature of Elements with Atomic Number > 100
- Electronic Configuration of Elements & Periodic Table
- Electronic Configurations & Types of Elements: s, p, d & f-block Elements
- Periodic Trends in Properties of Elements
- Metals, Non-Metals & Metalloids
These links will help you access specific resources and study materials for each topic within the chapter "Classification of Elements & Periodicity in Properties."
|
114 videos|263 docs|74 tests
|
FAQs on How to Master Classification of Elements & Periodicity in Properties for NEET? - Chemistry Class 11
| 1. What is the significance of the Modern Periodic Law in understanding element properties? |  |
| 2. How do I successfully memorize the electronic configurations of elements for NEET? |  |
| 3. What are the periodic trends I should focus on for NEET preparation? |  |
| 4. How do I differentiate between metals, non-metals, and metalloids effectively? |  |
| 5. What is the best way to revise the concepts of classification of elements and periodicity before the exam? |  |

















