All Exams >
NEET >
Weekly Tests for NEET Preparation >
All Questions
All questions of June Week 3 for NEET Exam
Among the elements with atomic numbers 9, 12, 16 and 36 which is highly electropositive?- a)Element with atomic number 9
- b)Element \vith atomic number 12
- c)Element with atomic number 16
- d)Element with atomic number 36
Correct answer is option 'B'. Can you explain this answer?
Among the elements with atomic numbers 9, 12, 16 and 36 which is highly electropositive?
a)
Element with atomic number 9
b)
Element \vith atomic number 12
c)
Element with atomic number 16
d)
Element with atomic number 36
|
|
Pooja Deshpande answered |
Introduction:
In order to determine which element is highly electropositive among the elements with atomic numbers 9, 12, 16, and 36, we need to understand the concept of electronegativity and how it relates to electropositivity. Electronegativity is a measure of an atom's ability to attract electrons towards itself in a chemical bond. Electropositivity, on the other hand, is the tendency of an atom to lose electrons and form positive ions. Therefore, an element with low electronegativity and high electropositivity will readily lose electrons and form positive ions.
Explanation:
Let's analyze each element and its electropositivity based on their atomic numbers:
Element with atomic number 9 (Fluorine):
Fluorine is a halogen and has a high electronegativity. It readily gains an electron to achieve a stable electron configuration. Therefore, it is not highly electropositive.
Element with atomic number 12 (Magnesium):
Magnesium is an alkaline earth metal and has a low electronegativity. It readily loses its two valence electrons to achieve a stable electron configuration. Therefore, it is highly electropositive.
Element with atomic number 16 (Sulfur):
Sulfur is a nonmetal and has a moderate electronegativity. It can gain or lose electrons depending on the reaction conditions. However, it is not highly electropositive as it tends to gain electrons to achieve a stable electron configuration.
Element with atomic number 36 (Krypton):
Krypton is a noble gas and has a very high electronegativity. It has a stable electron configuration and tends not to lose or gain electrons. Therefore, it is not highly electropositive.
Conclusion:
Based on the analysis, the element with atomic number 12 (Magnesium) is highly electropositive among the elements with atomic numbers 9, 12, 16, and 36. It has a low electronegativity and readily loses its two valence electrons to form a stable 2+ ion.
In order to determine which element is highly electropositive among the elements with atomic numbers 9, 12, 16, and 36, we need to understand the concept of electronegativity and how it relates to electropositivity. Electronegativity is a measure of an atom's ability to attract electrons towards itself in a chemical bond. Electropositivity, on the other hand, is the tendency of an atom to lose electrons and form positive ions. Therefore, an element with low electronegativity and high electropositivity will readily lose electrons and form positive ions.
Explanation:
Let's analyze each element and its electropositivity based on their atomic numbers:
Element with atomic number 9 (Fluorine):
Fluorine is a halogen and has a high electronegativity. It readily gains an electron to achieve a stable electron configuration. Therefore, it is not highly electropositive.
Element with atomic number 12 (Magnesium):
Magnesium is an alkaline earth metal and has a low electronegativity. It readily loses its two valence electrons to achieve a stable electron configuration. Therefore, it is highly electropositive.
Element with atomic number 16 (Sulfur):
Sulfur is a nonmetal and has a moderate electronegativity. It can gain or lose electrons depending on the reaction conditions. However, it is not highly electropositive as it tends to gain electrons to achieve a stable electron configuration.
Element with atomic number 36 (Krypton):
Krypton is a noble gas and has a very high electronegativity. It has a stable electron configuration and tends not to lose or gain electrons. Therefore, it is not highly electropositive.
Conclusion:
Based on the analysis, the element with atomic number 12 (Magnesium) is highly electropositive among the elements with atomic numbers 9, 12, 16, and 36. It has a low electronegativity and readily loses its two valence electrons to form a stable 2+ ion.
A monkey of mass 40 kg climbs on a rope which can stand a maximum tension of 600 N. In which of the following cases will the rope break. When the monkey
(a) Climbs up with an acceleration of 6 ms−2.
- a)640 N
- b)632 N
- c)760 N
- d)740 N
Correct answer is option 'A'. Can you explain this answer?
A monkey of mass 40 kg climbs on a rope which can stand a maximum tension of 600 N. In which of the following cases will the rope break. When the monkey
(a) Climbs up with an acceleration of 6 ms−2.
(a) Climbs up with an acceleration of 6 ms−2.
a)
640 N
b)
632 N
c)
760 N
d)
740 N
|
|
Preeti Iyer answered |
Mass of the monkey, m = 40 kg
Acceleration due to gravity, g = 10 m/s
Maximum tension that the rope can bear, Tmax = 600 N
Acceleration of the monkey, a = 6 m/s2 upward
Using Newtons second law of motion, we can write the equation of motion as:
T mg = ma
T = m(g + a)
= 40 (10 + 6)
= 640 N
Since T > Tmax, the rope will break in this case.
Acceleration due to gravity, g = 10 m/s
Maximum tension that the rope can bear, Tmax = 600 N
Acceleration of the monkey, a = 6 m/s2 upward
Using Newtons second law of motion, we can write the equation of motion as:
T mg = ma
T = m(g + a)
= 40 (10 + 6)
= 640 N
Since T > Tmax, the rope will break in this case.
A man of mass 70 kg stands on a weighing scale in a lift which is moving upwards with a uniform speed of 10 m s−1, what would be the reading on the scale?- a)105 kg
- b)75 kg
- c)70 kg
- d)35 kg
Correct answer is option 'C'. Can you explain this answer?
A man of mass 70 kg stands on a weighing scale in a lift which is moving upwards with a uniform speed of 10 m s−1, what would be the reading on the scale?
a)
105 kg
b)
75 kg
c)
70 kg
d)
35 kg
|
|
Pooja Shah answered |
Mass of the man, m = 70 kg
Acceleration, a = 0
Using Newton’s second law of motion, We can write the equation of motion as,
R – mg = ma
∴ R = mg = 70 × 10 = 700 N
∴ the weighing scale = 700 / g = 700 / 10 = 70 kg
Acceleration, a = 0
Using Newton’s second law of motion, We can write the equation of motion as,
R – mg = ma
∴ R = mg = 70 × 10 = 700 N
∴ the weighing scale = 700 / g = 700 / 10 = 70 kg
Newton’s third law states that when two bodies interact.- a)they exert forces on each other that at each instant are equal in magnitude and opposite in direction
- b)they exert forces on each other that at each instant are equal in magnitude and same in direction
- c)they exert forces on each other that at some instants are equal in magnitude and opposite in direction
- d)they exert forces on each other that at some instants are equal in magnitude and same in direction
Correct answer is option 'A'. Can you explain this answer?
Newton’s third law states that when two bodies interact.
a)
they exert forces on each other that at each instant are equal in magnitude and opposite in direction
b)
they exert forces on each other that at each instant are equal in magnitude and same in direction
c)
they exert forces on each other that at some instants are equal in magnitude and opposite in direction
d)
they exert forces on each other that at some instants are equal in magnitude and same in direction
|
|
Krishna Iyer answered |
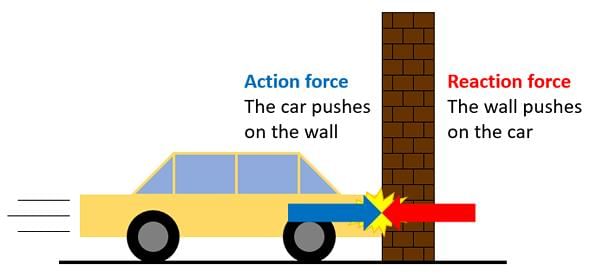
- The third law states that all forces between two objects exist in equal magnitude and opposite direction.
- If one object A exerts a force FA on a second object B, then B simultaneously exerts a force FB on A, and the two forces are equal in magnitude and opposite in direction, FA = −FB
- Newton's third Law:
If second law is applied to a rigid body- a)the acceleration is that of the centre of mass
- b)the acceleration is the average of all particles in the body
- c)the acceleration is that of any particle in the body
- d)none of the above
Correct answer is option 'A'. Can you explain this answer?
If second law is applied to a rigid body
a)
the acceleration is that of the centre of mass
b)
the acceleration is the average of all particles in the body
c)
the acceleration is that of any particle in the body
d)
none of the above
|
|
Geetika Shah answered |
The net external force on the rigid body is always equal to the total mass times the translational acceleration (i.e., Newton's second law holds for the translational motion, even when the net external torque is nonzero, and/or the body rotates).
A constant retarding force of 50 N is applied to a body of mass 20 kg moving initially with a speed of 15 m s−1How long does the body take to stop?- a)1.7.0 s
- b)6.0 s
- c)5.0 s
- d)none
Correct answer is option 'B'. Can you explain this answer?
A constant retarding force of 50 N is applied to a body of mass 20 kg moving initially with a speed of 15 m s−1How long does the body take to stop?
a)
1.7.0 s
b)
6.0 s
c)
5.0 s
d)
none
|
|
Lavanya Menon answered |
According to Newton's second Law
Force = mass x accleration
F = m.a
= m.v/t
t = m.v/F
= 20x15/50
= 6 sec
Force = mass x accleration
F = m.a
= m.v/t
t = m.v/F
= 20x15/50
= 6 sec
A monkey of mass 40 kg climbs on a rope which can stand a maximum tension of 600 N. What is the tension in the rope if the monkey climbs down with an acceleration of 4 ms−2- a)200 N
- b)300 N
- c)240 N
- d)280 N
Correct answer is option 'C'. Can you explain this answer?
A monkey of mass 40 kg climbs on a rope which can stand a maximum tension of 600 N. What is the tension in the rope if the monkey climbs down with an acceleration of 4 ms−2
a)
200 N
b)
300 N
c)
240 N
d)
280 N
|
|
Lavanya Menon answered |
Mass of the monkey, m = 40 kg
Acceleration due to gravity, g = 10 m/s
Maximum tension that the rope can bear, Tmax = 600 N
Acceleration of the monkey, a = 4 m/s2 downward
Using Newton’s second law of motion, we can write the equation of motion as:
mg T = ma
T = m (g- a)
= 40(10-4)
= 240 N
Acceleration due to gravity, g = 10 m/s
Maximum tension that the rope can bear, Tmax = 600 N
Acceleration of the monkey, a = 4 m/s2 downward
Using Newton’s second law of motion, we can write the equation of motion as:
mg T = ma
T = m (g- a)
= 40(10-4)
= 240 N
Two bodies of masses 10 kg and 20 kg respectively kept on a smooth, horizontal surface are tied to the ends of a light string. a horizontal force F = 600 N is applied to the 20 kg mass so as to pull it. What is the tension in the string?- a)250 N
- b)300 N
- c)150 N
- d)200 N
Correct answer is option 'D'. Can you explain this answer?
Two bodies of masses 10 kg and 20 kg respectively kept on a smooth, horizontal surface are tied to the ends of a light string. a horizontal force F = 600 N is applied to the 20 kg mass so as to pull it. What is the tension in the string?
a)
250 N
b)
300 N
c)
150 N
d)
200 N
|
|
Lavanya Menon answered |
For some instance assume both masses as one system, thus we get that
600 = 30a
Where a is the common acceleration of the system.
Now if we consider the 10kg block we get that
T = 10a
And a = 20m/s2
Thus we get T = 200N
600 = 30a
Where a is the common acceleration of the system.
Now if we consider the 10kg block we get that
T = 10a
And a = 20m/s2
Thus we get T = 200N
Diploblastic and triplo blastic are terms that describe- a)the number of in vaginations during embryonic development
- b)the number of heads during embryonic development
- c)the number of germinal layers during embryonic development
- d)the number of cell types during development
Correct answer is option 'C'. Can you explain this answer?
Diploblastic and triplo blastic are terms that describe
a)
the number of in vaginations during embryonic development
b)
the number of heads during embryonic development
c)
the number of germinal layers during embryonic development
d)
the number of cell types during development
|
|
Pooja Mukherjee answered |
Germinal Layers in Embryonic Development and Diploblastic/Triploblastic Classification
Embryonic development is a complex process that involves the formation of various tissues and organs from a single cell. During this process, the embryo undergoes several stages of development that are marked by the formation of germinal layers. Germinal layers are the layers of cells that differentiate into specific tissues and organs during embryonic development. These layers form the basis of the body plan of an organism and are classified as either diploblastic or triploblastic.
Diploblastic Organisms
Diploblastic organisms are those that have only two germinal layers: the ectoderm and endoderm. These layers are formed during gastrulation, which is the process by which the embryo folds in on itself to form a hollow ball of cells called the gastrula. In diploblastic organisms, the ectoderm gives rise to the outer layer of the body and the nervous system, while the endoderm gives rise to the inner layer of the body.
Examples of diploblastic organisms include cnidarians (e.g. jellyfish, corals, sea anemones) and ctenophores (comb jellies).
Triploblastic Organisms
Triploblastic organisms are those that have three germinal layers: the ectoderm, mesoderm, and endoderm. These layers are formed during gastrulation, but unlike in diploblastic organisms, the mesoderm layer is also formed. The mesoderm gives rise to the muscles, circulatory system, and other internal organs.
Examples of triploblastic organisms include most animals, including humans.
Conclusion
The diploblastic/triploblastic classification is based on the number of germinal layers that are present during embryonic development. Diploblastic organisms have two germinal layers (ectoderm and endoderm), while triploblastic organisms have three germinal layers (ectoderm, mesoderm, and endoderm). This classification is important for understanding the basic body plan of organisms and how different tissues and organs are formed during embryonic development.
Embryonic development is a complex process that involves the formation of various tissues and organs from a single cell. During this process, the embryo undergoes several stages of development that are marked by the formation of germinal layers. Germinal layers are the layers of cells that differentiate into specific tissues and organs during embryonic development. These layers form the basis of the body plan of an organism and are classified as either diploblastic or triploblastic.
Diploblastic Organisms
Diploblastic organisms are those that have only two germinal layers: the ectoderm and endoderm. These layers are formed during gastrulation, which is the process by which the embryo folds in on itself to form a hollow ball of cells called the gastrula. In diploblastic organisms, the ectoderm gives rise to the outer layer of the body and the nervous system, while the endoderm gives rise to the inner layer of the body.
Examples of diploblastic organisms include cnidarians (e.g. jellyfish, corals, sea anemones) and ctenophores (comb jellies).
Triploblastic Organisms
Triploblastic organisms are those that have three germinal layers: the ectoderm, mesoderm, and endoderm. These layers are formed during gastrulation, but unlike in diploblastic organisms, the mesoderm layer is also formed. The mesoderm gives rise to the muscles, circulatory system, and other internal organs.
Examples of triploblastic organisms include most animals, including humans.
Conclusion
The diploblastic/triploblastic classification is based on the number of germinal layers that are present during embryonic development. Diploblastic organisms have two germinal layers (ectoderm and endoderm), while triploblastic organisms have three germinal layers (ectoderm, mesoderm, and endoderm). This classification is important for understanding the basic body plan of organisms and how different tissues and organs are formed during embryonic development.
K+ and Cl- ions are isoelectronic. Which of the statements is not correct?
- a)Both K+ and Cl- ions contain 18 electrons
- b)Both K+ and Cl- ions have same configuration
- c)K+ ion is bigger than Cl- ion in ionic size
- d)Cl- ion is bigger than K+ ion in size
Correct answer is option 'C'. Can you explain this answer?
K+ and Cl- ions are isoelectronic. Which of the statements is not correct?
a)
Both K+ and Cl- ions contain 18 electrons
b)
Both K+ and Cl- ions have same configuration
c)
K+ ion is bigger than Cl- ion in ionic size
d)
Cl- ion is bigger than K+ ion in size
|
|
Ajay Yadav answered |

According to second law of motion- a)The momentum of a body is directly proportional to the applied force and takes place in the direction in which the force acts
- b)The change of momentum of a body is directly proportional to the applied force and takes place in the direction in which the force acts
- c)The rate of change of momentum of a body is directly proportional to the applied force and takes place in the direction in which the force acts
- d)The momentum of a body is directly proportional to the applied force and takes place in the direction opposite to the force
Correct answer is option 'C'. Can you explain this answer?
According to second law of motion
a)
The momentum of a body is directly proportional to the applied force and takes place in the direction in which the force acts
b)
The change of momentum of a body is directly proportional to the applied force and takes place in the direction in which the force acts
c)
The rate of change of momentum of a body is directly proportional to the applied force and takes place in the direction in which the force acts
d)
The momentum of a body is directly proportional to the applied force and takes place in the direction opposite to the force
|
|
Vijay Bansal answered |
According to Newton s Second Law of Motion, also known as the Law of Force and Acceleration, a force upon an object causes it to accelerate according to the formula net force = mass x acceleration. So the acceleration of the object is directly proportional to the force and inversely proportional to the mass.
A stone of mass 0.25 kg tied to the end of a string is whirled round in a circle of radius 1.5 m with a speed of 40 rev./min in a horizontal plane. The speed is then increased beyond the maximum permissible value, and the string breaks suddenly, which of the following correctly describes the trajectory of the stone after the string breaks- a)the stone flies off at an angle with the tangent whose magnitude depends on the speed of the particle.
- b)the stone moves radially inwards
- c)the stone flies off tangentially from the instant the string breaks
- d)the stone moves radially outwards
Correct answer is 'C'. Can you explain this answer?
A stone of mass 0.25 kg tied to the end of a string is whirled round in a circle of radius 1.5 m with a speed of 40 rev./min in a horizontal plane. The speed is then increased beyond the maximum permissible value, and the string breaks suddenly, which of the following correctly describes the trajectory of the stone after the string breaks
a)
the stone flies off at an angle with the tangent whose magnitude depends on the speed of the particle.
b)
the stone moves radially inwards
c)
the stone flies off tangentially from the instant the string breaks
d)
the stone moves radially outwards
|
|
Pratul Garg answered |
C.because when the string breaks centripetal force is no longer there so the velocity in circular motion is no longer there so the stone flies off tangentially from the point of it's breaking
A man of mass 70 kg stands on a weighing scale in a lift which is moving upwards with a uniform acceleration of 10 m s−2 what would be the reading on the scale?- a)150 kg
- b)120 kg
- c)130 kg
- d)140 kg
Correct answer is option 'D'. Can you explain this answer?
A man of mass 70 kg stands on a weighing scale in a lift which is moving upwards with a uniform acceleration of 10 m s−2 what would be the reading on the scale?
a)
150 kg
b)
120 kg
c)
130 kg
d)
140 kg
|
|
Raghav Bansal answered |
According to Newton's second Law of motion,
Apparent weight, R = m(a+g)
Apparent weight, R = m(a+g)
= 70(10+10)
= 1400N
mass = R/g
= 1400/10
= 140Kg
Give the magnitude and direction of the net force acting on a stone of mass 0.1 kg lying on the floor of a train which is accelerating with 1 ms−2, the stone being at rest relative to the train. Neglect air resistance.
- a)1.0 N in the direction of motion
- b)0.1 N opposite to the direction of motion
- c)0.1 N along the direction of motion
- d)0.2 opposite to the direction of motion
Correct answer is option 'C'. Can you explain this answer?
Give the magnitude and direction of the net force acting on a stone of mass 0.1 kg lying on the floor of a train which is accelerating with 1 ms−2, the stone being at rest relative to the train. Neglect air resistance.
a)
1.0 N in the direction of motion
b)
0.1 N opposite to the direction of motion
c)
0.1 N along the direction of motion
d)
0.2 opposite to the direction of motion
|
|
Pooja Shah answered |
Weight of the stone is balanced by the reaction of the floor. The only acceleration is provided by the horizontal motion of the train.
a = 1ms-2
Force in horizontal direction
F = ma = 0.1 x 1 = 0.1N
Which of the following statements is incorrect with regard to bilateral symmetry?
- a)Body can be divided into two equal halves by a single plane only.
- b)The organisms that show bilateral symmetry have paired body organs that occur on the two sides of a central axis.
- c)It is found in all invertebrates and few vertebrates.
- d)Spider and crab show bilateral symmetry.
Correct answer is option 'C'. Can you explain this answer?
Which of the following statements is incorrect with regard to bilateral symmetry?
a)
Body can be divided into two equal halves by a single plane only.
b)
The organisms that show bilateral symmetry have paired body organs that occur on the two sides of a central axis.
c)
It is found in all invertebrates and few vertebrates.
d)
Spider and crab show bilateral symmetry.
|
|
Poulomi Roy answered |
Bilateral Symmetry
Bilateral symmetry refers to the arrangement of body parts in such a way that an organism can be divided into two equal halves by a single plane passing through the central axis of the body. This type of symmetry is found in many animals, especially invertebrates and some vertebrates.
Incorrect statement
The incorrect statement is option C, which states that bilateral symmetry is found in all invertebrates and few vertebrates. This statement is incorrect because bilateral symmetry is actually found in the majority of animals, both invertebrates, and vertebrates. Almost all animals belonging to the phyla Chordata, Arthropoda, Mollusca, and Annelida exhibit bilateral symmetry.
Examples of animals with bilateral symmetry
- Invertebrates like insects, crustaceans, spiders, and worms exhibit bilateral symmetry.
- Vertebrates like fish, amphibians, reptiles, birds, and mammals exhibit bilateral symmetry.
Advantages of bilateral symmetry
Bilateral symmetry provides several advantages to animals:
- It allows for more efficient movement and coordination since paired limbs and muscles can work together to produce more precise movements.
- It facilitates the development of specialized organs and structures, such as eyes and ears, that are paired and located on opposite sides of the body.
- It enables animals to have directional movement, since paired limbs can be used to move forward or backward, up or down, and left or right.
Conclusion
Bilateral symmetry is a common type of symmetry found in the majority of animals, both invertebrates, and vertebrates. It allows for more efficient movement and coordination, facilitates the development of specialized organs and structures, and enables directional movement.
Bilateral symmetry refers to the arrangement of body parts in such a way that an organism can be divided into two equal halves by a single plane passing through the central axis of the body. This type of symmetry is found in many animals, especially invertebrates and some vertebrates.
Incorrect statement
The incorrect statement is option C, which states that bilateral symmetry is found in all invertebrates and few vertebrates. This statement is incorrect because bilateral symmetry is actually found in the majority of animals, both invertebrates, and vertebrates. Almost all animals belonging to the phyla Chordata, Arthropoda, Mollusca, and Annelida exhibit bilateral symmetry.
Examples of animals with bilateral symmetry
- Invertebrates like insects, crustaceans, spiders, and worms exhibit bilateral symmetry.
- Vertebrates like fish, amphibians, reptiles, birds, and mammals exhibit bilateral symmetry.
Advantages of bilateral symmetry
Bilateral symmetry provides several advantages to animals:
- It allows for more efficient movement and coordination since paired limbs and muscles can work together to produce more precise movements.
- It facilitates the development of specialized organs and structures, such as eyes and ears, that are paired and located on opposite sides of the body.
- It enables animals to have directional movement, since paired limbs can be used to move forward or backward, up or down, and left or right.
Conclusion
Bilateral symmetry is a common type of symmetry found in the majority of animals, both invertebrates, and vertebrates. It allows for more efficient movement and coordination, facilitates the development of specialized organs and structures, and enables directional movement.
A train runs along an unbanked circular track of radius 30 m at a speed of 54 km/h. The mass of the train is 106 kg. What is the angle of banking required to prevent wearing out of the rail ?
- a)27∘
- b)36.8∘
- c)42∘
- d)32∘
Correct answer is option 'B'. Can you explain this answer?
A train runs along an unbanked circular track of radius 30 m at a speed of 54 km/h. The mass of the train is 106 kg. What is the angle of banking required to prevent wearing out of the rail ?
a)
27∘
b)
36.8∘
c)
42∘
d)
32∘
|
|
Tanishq Tiwari answered |
Given:
Radius of the circular track, r = 30 m
Speed of the train, v = 54 km/h = 15 m/s
Mass of the train, m = 106 kg
To find:
Angle of banking required to prevent wearing out of the rail.
Solution:
Let θ be the angle of banking required.
The centripetal force required for the train to move in a circular path is given by,
F = mv²/r
where,
m = mass of the train
v = velocity of the train
r = radius of the circular track
The gravitational force acting on the train is given by,
mg
where,
m = mass of the train
g = acceleration due to gravity
The normal force acting on the train is given by,
N = mg cosθ
where,
θ = angle of banking
mg sinθ = mv²/r
sinθ = v²/(rg) = (15²)/(30×9.8) = 0.7653
θ = sin⁻¹(0.7653) = 37°
Therefore, the angle of banking required to prevent wearing out of the rail is 37°. Answer: (b)
Radius of the circular track, r = 30 m
Speed of the train, v = 54 km/h = 15 m/s
Mass of the train, m = 106 kg
To find:
Angle of banking required to prevent wearing out of the rail.
Solution:
Let θ be the angle of banking required.
The centripetal force required for the train to move in a circular path is given by,
F = mv²/r
where,
m = mass of the train
v = velocity of the train
r = radius of the circular track
The gravitational force acting on the train is given by,
mg
where,
m = mass of the train
g = acceleration due to gravity
The normal force acting on the train is given by,
N = mg cosθ
where,
θ = angle of banking
mg sinθ = mv²/r
sinθ = v²/(rg) = (15²)/(30×9.8) = 0.7653
θ = sin⁻¹(0.7653) = 37°
Therefore, the angle of banking required to prevent wearing out of the rail is 37°. Answer: (b)
Rolling friction is- a)greater than or equal to static
- b)greater than static friction
- c)equal to static friction
- d)less than static friction
Correct answer is option 'D'. Can you explain this answer?
Rolling friction is
a)
greater than or equal to static
b)
greater than static friction
c)
equal to static friction
d)
less than static friction
|
|
Geetika Shah answered |
Rolling friction is always less than static friction because in order for an object to roll the force of friction between it and surface must be large enough to keep the object from sliding. Hence rolling friction is always greater than static force.
Which of the following elements will have highest ionisation energy?- a)1s2 2s2 2p6 3s1
- b)1s2 2s2 2p6 3s2 3p3
- c)1s2 2s2 2p6 3s2 3p4
- d)1s2 2s2 2p6 3s2 3p1
Correct answer is option 'B'. Can you explain this answer?
Which of the following elements will have highest ionisation energy?
a)
1s2 2s2 2p6 3s1
b)
1s2 2s2 2p6 3s2 3p3
c)
1s2 2s2 2p6 3s2 3p4
d)
1s2 2s2 2p6 3s2 3p1
|
|
Jaspreet answered |
In half filled and fully filled orbitals it's difficult to remove an electron from its outermost shell cause it's Highly stable electronic configuration so it's option B !!!
Read the given statements and select the correct option.
Statement 1: All triploblastic animals are eucoelomates.
Statement 2: They have a false coelom.
- a)Both statements 1 and 2 are correct and statement 2 is the correct explanation of statement 1.
- b)Both statements 1 and 2 are correct but statement 2 is not the correct explanation of statement 1.
- c)Statement 1 is correct and statement 2 is incorrect.
- d)Both statements 1 and 2 are incorrect.
Correct answer is option 'D'. Can you explain this answer?
Read the given statements and select the correct option.
Statement 1: All triploblastic animals are eucoelomates.
Statement 2: They have a false coelom.
Statement 1: All triploblastic animals are eucoelomates.
Statement 2: They have a false coelom.
a)
Both statements 1 and 2 are correct and statement 2 is the correct explanation of statement 1.
b)
Both statements 1 and 2 are correct but statement 2 is not the correct explanation of statement 1.
c)
Statement 1 is correct and statement 2 is incorrect.
d)
Both statements 1 and 2 are incorrect.
|
|
Geetika Shah answered |
- Animals that belong to the phylum Platyhelminthes to Chordata are all triploblastic.
- But Platyhelminthes and aschelminthes belong to pseudocoelomates.
- Annelids, arthropods, echinoderms and chordates are under eucoelomates. This means not all triploblastic animals are eucoelomates.
- Pseudocoelomates have false coelom while eucoelomate animals only have the true coelom.
Hence, both statement 1 and statement 2 are incorrect.
A rocket with a lift-off mass 20,000 kg is blasted upwards with an initial acceleration of 5.0 ms-2. Calculate the initial thrust (force) of the blast.- a)365000 N
- b)378000 N
- c)300000 N
- d)350000 N
Correct answer is option 'C'. Can you explain this answer?
A rocket with a lift-off mass 20,000 kg is blasted upwards with an initial acceleration of 5.0 ms-2. Calculate the initial thrust (force) of the blast.
a)
365000 N
b)
378000 N
c)
300000 N
d)
350000 N

|
Anisha Chauhan answered |
Explanation:Initial thrust = upthrust required to impart acceleration + uthrust to overcome gravity
=ma+mg = m(a+g) = 20000(5+10) = 300000 N
An element X has atomic number 19. What will be the formula of its oxide?- a)X2O
- b)XO
- c)XO2
- d)X2O3
Correct answer is option 'A'. Can you explain this answer?
An element X has atomic number 19. What will be the formula of its oxide?
a)
X2O
b)
XO
c)
XO2
d)
X2O3
|
|
Mira Joshi answered |
Element X has atomic number 19. Its valency will be one. Hence, the formula ofits oxide will be X2O.
Z = 19; 1s22s22p63s23p64s1
Z = 19; 1s22s22p63s23p64s1
According to first law of motion- a)body acted on by no net force moves with increasing velocity and negative acceleration
- b)body acted on by no net force moves with constant velocity (which may be zero) and zero acceleration
- c)body acted on by net force moves with constant velocity (which may be zero)
- d)body acted on by zero net force moves with non zero acceleration
Correct answer is option 'B'. Can you explain this answer?
According to first law of motion
a)
body acted on by no net force moves with increasing velocity and negative acceleration
b)
body acted on by no net force moves with constant velocity (which may be zero) and zero acceleration
c)
body acted on by net force moves with constant velocity (which may be zero)
d)
body acted on by zero net force moves with non zero acceleration
|
|
Rohan Singh answered |
Newton's first law of motion states that an object at rest remains at rest and an object in motion remains in motion with the same velocity unless acted upon by what we call an unbalanced force. Let's break this law down by defining some key terms. Velocity is the speed of an object in a specified direction. An unbalanced force is an external force that changes the motion of an object. When an object is at rest or moving at a constant velocity, all the forces acting on it are balanced.
For example, my cup of coffee is resting on my kitchen table as I make this lesson. The force of gravity pulling down is balanced by the force of my kitchen table pushing up on my cup of coffee. Now, if I am to move my coffee, I must apply an external force to disrupt the balance. Thank goodness my muscles are strong enough to apply such a force so I can enjoy my cup of coffee. Ah, now that's good joe! Newton's first law of motion is also referred to as the law of inertia. Inertia is simply the resistance to change in motion. In short, objects tend to keep doing what they are already doing.
Static friction force- a)increases until the body starts to move
- b)decreases before moving and increases later
- c)remains constant
- d)is a strong interaction force
Correct answer is option 'A'. Can you explain this answer?
Static friction force
a)
increases until the body starts to move
b)
decreases before moving and increases later
c)
remains constant
d)
is a strong interaction force

|
Priyanka Roy answered |
Explanation:It is the maximum friction, where a body just starts to move over the surface. for example. A large block of mass m is placed on a horizontal table. Apply a small force. the block does not move due to the friction force which balances the applied force. Now Gradually increase the applied force untill it start moving. This maximum applied force acting in opposite direction is called as Static Friction.
When several forces act on a body- a)the effect on its motion is the same as when a single force acts on the body
- b)the effect on its motion is the same as when a single force, equal to the vector sum (resultant) of the forces, acts on the body
- c)the effect on its motion is the same as when a single force, not equal to the vector sum (resultant) of the forces, acts on the body
- d)none
Correct answer is option 'B'. Can you explain this answer?
When several forces act on a body
a)
the effect on its motion is the same as when a single force acts on the body
b)
the effect on its motion is the same as when a single force, equal to the vector sum (resultant) of the forces, acts on the body
c)
the effect on its motion is the same as when a single force, not equal to the vector sum (resultant) of the forces, acts on the body
d)
none
|
|
Raghav Bansal answered |
When an object has several forces acting on it, the effect of force is the same as one force acting on the object in a certain direction and this overall force is called the ‘resultant force’. The resultant force is essential to change the velocity of an object.
Select the correct matching of animals, their symmetry, organisation and coelom type.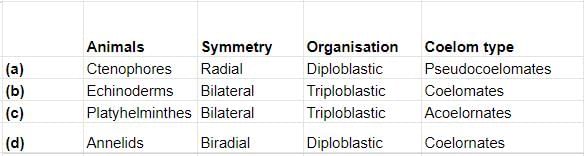
- a)a
- b)b
- c)c
- d)d
Correct answer is option 'C'. Can you explain this answer?
Select the correct matching of animals, their symmetry, organisation and coelom type.

a)
a
b)
b
c)
c
d)
d
|
|
Jyoti Sengupta answered |
Platyhelminthes are triploblastic, acoelomate organism showing bilateral symmetry. Ctenophores have borads symmetry, are acoelomates having diploblastic organisation. In echinoderms, larvae have bilateral symmetry whiled add have radial symmetry. They are coelomates with triplobla organisation. Annelids are triploblastic, coelomaterorganism showing bilateral symmetry.
Choose the incorrect statement.- a)Chemical reactivity tends to be high in group 1 metals, lower in elements in middle and increases to maximum in the group 17
- b)Halogens have very high negative electron gain enthalpy
- c)Noble gases have large positive electron gain enthalpy
- d)Decrease in electronegativities across a period is accompanied by an increase in non-metallic properties
Correct answer is option 'D'. Can you explain this answer?
Choose the incorrect statement.
a)
Chemical reactivity tends to be high in group 1 metals, lower in elements in middle and increases to maximum in the group 17
b)
Halogens have very high negative electron gain enthalpy
c)
Noble gases have large positive electron gain enthalpy
d)
Decrease in electronegativities across a period is accompanied by an increase in non-metallic properties
|
|
Meera Singh answered |
Electronegativity increases across a period.
In the periodic table, the maximum chemical reactivity is at the extreme left (alkali metals) and extreme right (halogens). Which properties of these two groups are responsible for this?- a)Least ionisation enthalpy on the left and highest negative electron gain enthalpy on the right
- b)Non-metallic character on the left and metallic character on the right
- c)High atomic radii on the left and small atomic radii on the right
- d)Highest electronegativity on the left and least electronegativity oh the right
Correct answer is option 'A'. Can you explain this answer?
In the periodic table, the maximum chemical reactivity is at the extreme left (alkali metals) and extreme right (halogens). Which properties of these two groups are responsible for this?
a)
Least ionisation enthalpy on the left and highest negative electron gain enthalpy on the right
b)
Non-metallic character on the left and metallic character on the right
c)
High atomic radii on the left and small atomic radii on the right
d)
Highest electronegativity on the left and least electronegativity oh the right
|
|
Prisha Rane answered |
Ionization enthalpy and electron gain enthalpy:
- The alkali metals on the extreme left of the periodic table have the lowest ionization enthalpies, making it easier for them to lose electrons and become positively charged ions.
- The halogens on the extreme right have the highest negative electron gain enthalpies, making them highly reactive as they tend to gain electrons to achieve a stable electron configuration.
Non-metallic and metallic character:
- Alkali metals exhibit non-metallic character as they readily lose electrons to form positively charged ions.
- Halogens exhibit metallic character as they tend to gain electrons to achieve a stable electron configuration.
Atomic radii:
- Alkali metals have relatively high atomic radii due to the presence of more electron shells.
- Halogens have small atomic radii as they have a higher effective nuclear charge, pulling the electrons closer to the nucleus.
Electronegativity:
- Alkali metals have the lowest electronegativity as they readily lose electrons.
- Halogens have the highest electronegativity as they tend to gain electrons.
In conclusion, the extreme left (alkali metals) and extreme right (halogens) of the periodic table exhibit maximum chemical reactivity due to their properties of ionization enthalpy and electron gain enthalpy, non-metallic and metallic character, atomic radii, and electronegativity.
- The alkali metals on the extreme left of the periodic table have the lowest ionization enthalpies, making it easier for them to lose electrons and become positively charged ions.
- The halogens on the extreme right have the highest negative electron gain enthalpies, making them highly reactive as they tend to gain electrons to achieve a stable electron configuration.
Non-metallic and metallic character:
- Alkali metals exhibit non-metallic character as they readily lose electrons to form positively charged ions.
- Halogens exhibit metallic character as they tend to gain electrons to achieve a stable electron configuration.
Atomic radii:
- Alkali metals have relatively high atomic radii due to the presence of more electron shells.
- Halogens have small atomic radii as they have a higher effective nuclear charge, pulling the electrons closer to the nucleus.
Electronegativity:
- Alkali metals have the lowest electronegativity as they readily lose electrons.
- Halogens have the highest electronegativity as they tend to gain electrons.
In conclusion, the extreme left (alkali metals) and extreme right (halogens) of the periodic table exhibit maximum chemical reactivity due to their properties of ionization enthalpy and electron gain enthalpy, non-metallic and metallic character, atomic radii, and electronegativity.
Given below are the names of few elements based on their position in the periodic table. Identify the element which is not correctly placed.- a)An element which tends to lose three electrons - Aluminium
- b)An element which tends to gain two electrons - Iodine
- c)An element with valency four - Silicon
- d)A transuranium element - Plutonium
Correct answer is option 'B'. Can you explain this answer?
Given below are the names of few elements based on their position in the periodic table. Identify the element which is not correctly placed.
a)
An element which tends to lose three electrons - Aluminium
b)
An element which tends to gain two electrons - Iodine
c)
An element with valency four - Silicon
d)
A transuranium element - Plutonium
|
|
Jyoti Sengupta answered |
Iodine can gain only one electron.
I + e- → I-
I + e- → I-
Ionic radius in a group while moving down.- a)Remains same from top to bottom
- b)Decreases from top to bottom
- c)Increases from top to bottom
- d)First increases and then decreases
Correct answer is option 'C'. Can you explain this answer?
Ionic radius in a group while moving down.
a)
Remains same from top to bottom
b)
Decreases from top to bottom
c)
Increases from top to bottom
d)
First increases and then decreases
|
|
Jyoti Sengupta answered |
Ionic radius in a group increases from top to bottom since one energy shell is added with each period
A sudden large jump between the values of second and third ionization energies of an element would be associated with which ofthe following electronic configuration?- a)1s2, 2s2, 2p6, 3s1, 3p2
- b)1s2, 2s2, 2p6, 3s2, 3p1
- c)1s2, 2s2, 2p6, 3s1
- d)1s2, 2s2, 2p6, 3s2
Correct answer is option 'D'. Can you explain this answer?
A sudden large jump between the values of second and third ionization energies of an element would be associated with which ofthe following electronic configuration?
a)
1s2, 2s2, 2p6, 3s1, 3p2
b)
1s2, 2s2, 2p6, 3s2, 3p1
c)
1s2, 2s2, 2p6, 3s1
d)
1s2, 2s2, 2p6, 3s2
|
|
Aarav Shah answered |
Understanding Ionization Energies
Ionization energy refers to the energy required to remove an electron from an atom. A sudden large jump between the second and third ionization energies indicates that removing the third electron requires significantly more energy than removing the first two. This typically occurs when the electron being removed is from a more stable electron configuration.
Analysis of Electronic Configurations
Let’s analyze the provided electronic configurations:
- Option A: 1s2, 2s2, 2p6, 3s1, 3p2
- Removal of the first electron from 3p2 is relatively easy. Removal of a second electron from the same subshell remains manageable. The third ionization would not show a large jump since the remaining electrons are still in the same energy levels.
- Option B: 1s2, 2s2, 2p6, 3s2, 3p1
- Similar to option A, removing the first two electrons from 3s2 and 3p1 does not reach a stable noble gas configuration. Hence, the third ionization does not exhibit a significant jump.
- Option C: 1s2, 2s2, 2p6, 3s1
- Removing one electron from 3s1 still doesn’t lead to a substantial increase in ionization energy for subsequent removals, as there’s no stable electron configuration left behind.
- Option D: 1s2, 2s2, 2p6, 3s2
- Here, the removal of the first two electrons from 3s2 would occur easily, leading to a fully filled 2p subshell (a stable configuration). The third ionization would require removing an electron from a new, higher energy subshell (3p) which is much more stable, resulting in a significant jump in ionization energy.
Conclusion
Thus, option D represents an electronic configuration where the large jump between the second and third ionization energies can be attributed to the stability of the noble gas configuration achieved after the removal of two electrons from the 3s subshell.
Ionization energy refers to the energy required to remove an electron from an atom. A sudden large jump between the second and third ionization energies indicates that removing the third electron requires significantly more energy than removing the first two. This typically occurs when the electron being removed is from a more stable electron configuration.
Analysis of Electronic Configurations
Let’s analyze the provided electronic configurations:
- Option A: 1s2, 2s2, 2p6, 3s1, 3p2
- Removal of the first electron from 3p2 is relatively easy. Removal of a second electron from the same subshell remains manageable. The third ionization would not show a large jump since the remaining electrons are still in the same energy levels.
- Option B: 1s2, 2s2, 2p6, 3s2, 3p1
- Similar to option A, removing the first two electrons from 3s2 and 3p1 does not reach a stable noble gas configuration. Hence, the third ionization does not exhibit a significant jump.
- Option C: 1s2, 2s2, 2p6, 3s1
- Removing one electron from 3s1 still doesn’t lead to a substantial increase in ionization energy for subsequent removals, as there’s no stable electron configuration left behind.
- Option D: 1s2, 2s2, 2p6, 3s2
- Here, the removal of the first two electrons from 3s2 would occur easily, leading to a fully filled 2p subshell (a stable configuration). The third ionization would require removing an electron from a new, higher energy subshell (3p) which is much more stable, resulting in a significant jump in ionization energy.
Conclusion
Thus, option D represents an electronic configuration where the large jump between the second and third ionization energies can be attributed to the stability of the noble gas configuration achieved after the removal of two electrons from the 3s subshell.
Force is required- a)to start a stationary object and to stop a moving object
- b)only to keep an object moving
- c)only to stop a moving object
- d)only to start a stationary object moving
Correct answer is option 'A'. Can you explain this answer?
Force is required
a)
to start a stationary object and to stop a moving object
b)
only to keep an object moving
c)
only to stop a moving object
d)
only to start a stationary object moving

|
Gowri Nambiar answered |
Explanation:force is required to start a stationary object and to stop a moving object due to inertia. Inertia is a property of matter by which it continues in its existing state of rest or uniform motion in a straight line, unless that state is changed by an external force. This is also called law of inertia or newton's first law of motion
When bodies are in contact with each other then - a)contact forces are equal and in same direction
- b)contact forces are not equal and in same direction
- c)contact forces are equal and opposite in direction
- d)contact forces are not equal and opposite in direction
Correct answer is option 'C'. Can you explain this answer?
When bodies are in contact with each other then
a)
contact forces are equal and in same direction
b)
contact forces are not equal and in same direction
c)
contact forces are equal and opposite in direction
d)
contact forces are not equal and opposite in direction

|
Gowri Nambiar answered |
Explanation:according to newton's third law of motion for every action there is always equal and opposite reaction or the mutual actions of two bodies upon each other. Whenever two objects interact with each other, then the pair of forces acting on them are equal in magnitude and opposite in direction.Contact forces are mutual forces (action and reaction)
Predict the formula of a compound formed by aluminium and sulphur.- a)Al2S2
- b)Al3S2
- c)Al2S3
- d)AIS
Correct answer is option 'C'. Can you explain this answer?
Predict the formula of a compound formed by aluminium and sulphur.
a)
Al2S2
b)
Al3S2
c)
Al2S3
d)
AIS
|
|
Shalini Sarkar answered |
The compound formed by aluminium and sulphur is aluminium sulphide (Al2S3).
Explanation:
1. Determine the oxidation states:
Aluminium typically has an oxidation state of +3 (Al3+), while sulphur typically has an oxidation state of -2 (S2-).
2. Determine the number of atoms needed to balance the charges:
Since the oxidation state of aluminium is +3 and the oxidation state of sulphur is -2, we need to balance these charges. This can be done by combining two aluminium ions with three sulphur ions.
3. Write the formula:
The formula for the compound formed by aluminium and sulphur is Al2S3. This indicates that two aluminium ions combine with three sulphur ions to form the compound.
4. Check the formula for consistency:
To confirm that the formula is consistent, we can determine the total charge of the compound. The charge of two aluminium ions (+6) should balance the charge of three sulphur ions (-6).
Summary:
The compound formed by aluminium and sulphur is aluminium sulphide (Al2S3). This formula indicates that two aluminium ions combine with three sulphur ions to form the compound. The oxidation state of aluminium is +3, and the oxidation state of sulphur is -2, so the charges are balanced in the compound.
Explanation:
1. Determine the oxidation states:
Aluminium typically has an oxidation state of +3 (Al3+), while sulphur typically has an oxidation state of -2 (S2-).
2. Determine the number of atoms needed to balance the charges:
Since the oxidation state of aluminium is +3 and the oxidation state of sulphur is -2, we need to balance these charges. This can be done by combining two aluminium ions with three sulphur ions.
3. Write the formula:
The formula for the compound formed by aluminium and sulphur is Al2S3. This indicates that two aluminium ions combine with three sulphur ions to form the compound.
4. Check the formula for consistency:
To confirm that the formula is consistent, we can determine the total charge of the compound. The charge of two aluminium ions (+6) should balance the charge of three sulphur ions (-6).
Summary:
The compound formed by aluminium and sulphur is aluminium sulphide (Al2S3). This formula indicates that two aluminium ions combine with three sulphur ions to form the compound. The oxidation state of aluminium is +3, and the oxidation state of sulphur is -2, so the charges are balanced in the compound.
The main reason for showing anomalous properties of the first member of a group in s or p-block is- a)Maximum chemical reactivity
- b)Maximum electronegativity and different configurations
- c)Small size, large charge/radius ratio
- d)Tendency to form multiple bonds
Correct answer is option 'C'. Can you explain this answer?
The main reason for showing anomalous properties of the first member of a group in s or p-block is
a)
Maximum chemical reactivity
b)
Maximum electronegativity and different configurations
c)
Small size, large charge/radius ratio
d)
Tendency to form multiple bonds
|
|
Dev Patel answered |
Due to small size, large charge/radius ratio and high electronegativity, the first elementshows anomalous behaviour.
Which of the following is correctly matched?- a)Radial symmetry - Coelenterates
- b)Coelomates - Aschelminthes
- c)Metamerism - Molluscs
- d)Triploblastic - Sponges
Correct answer is option 'A'. Can you explain this answer?
Which of the following is correctly matched?
a)
Radial symmetry - Coelenterates
b)
Coelomates - Aschelminthes
c)
Metamerism - Molluscs
d)
Triploblastic - Sponges
|
|
Ujwal Basu answered |
Radial symmetry is correctly matched with coelenterates.
Coelenterates, also known as Cnidarians, exhibit radial symmetry. Radial symmetry is a type of symmetry where the body parts are arranged around a central axis, similar to the spokes of a wheel. This arrangement allows the organism to be divided into equal halves in any plane passing through the central axis.
Coelenterates are a phylum of invertebrate animals that include jellyfish, sea anemones, and coral. They have a sac-like body plan with a central gastrovascular cavity. The body is organized around a central axis, with tentacles radiating outwards from the central region. These tentacles are armed with specialized stinging cells called cnidocytes, which they use for defense and prey capture.
Radial symmetry in coelenterates allows them to interact with their environment in all directions. They can capture prey from any direction, and their sensory structures, such as eyespots or statocysts, are distributed around the body. This symmetry is particularly advantageous for sessile or slow-moving organisms, as they can detect and respond to stimuli from any direction.
Other options in the question are incorrect matches:
- Coelomates are animals that possess a true coelom, a fluid-filled body cavity completely lined by mesoderm. Aschelminthes, also known as Nematodes, are pseudocoelomates, meaning they have a body cavity that is not completely lined by mesoderm. Therefore, option B is incorrect.
- Metamerism refers to the organization of the body into repeating segments or metameres. This characteristic is seen in annelids, such as earthworms, not molluscs. Therefore, option C is incorrect.
- Triploblastic refers to organisms that have three germ layers: ectoderm, mesoderm, and endoderm. Sponges, which belong to the phylum Porifera, are diploblastic, meaning they have only two germ layers. Therefore, option D is incorrect.
Coelenterates, also known as Cnidarians, exhibit radial symmetry. Radial symmetry is a type of symmetry where the body parts are arranged around a central axis, similar to the spokes of a wheel. This arrangement allows the organism to be divided into equal halves in any plane passing through the central axis.
Coelenterates are a phylum of invertebrate animals that include jellyfish, sea anemones, and coral. They have a sac-like body plan with a central gastrovascular cavity. The body is organized around a central axis, with tentacles radiating outwards from the central region. These tentacles are armed with specialized stinging cells called cnidocytes, which they use for defense and prey capture.
Radial symmetry in coelenterates allows them to interact with their environment in all directions. They can capture prey from any direction, and their sensory structures, such as eyespots or statocysts, are distributed around the body. This symmetry is particularly advantageous for sessile or slow-moving organisms, as they can detect and respond to stimuli from any direction.
Other options in the question are incorrect matches:
- Coelomates are animals that possess a true coelom, a fluid-filled body cavity completely lined by mesoderm. Aschelminthes, also known as Nematodes, are pseudocoelomates, meaning they have a body cavity that is not completely lined by mesoderm. Therefore, option B is incorrect.
- Metamerism refers to the organization of the body into repeating segments or metameres. This characteristic is seen in annelids, such as earthworms, not molluscs. Therefore, option C is incorrect.
- Triploblastic refers to organisms that have three germ layers: ectoderm, mesoderm, and endoderm. Sponges, which belong to the phylum Porifera, are diploblastic, meaning they have only two germ layers. Therefore, option D is incorrect.
Which of the following will have lowest electron affinity?- a)Nitrogen
- b)Oxygen
- c)Argon
- d)Boron
Correct answer is option 'C'. Can you explain this answer?
Which of the following will have lowest electron affinity?
a)
Nitrogen
b)
Oxygen
c)
Argon
d)
Boron
|
|
Shruti Chavan answered |
Understanding Electron Affinity
Electron affinity refers to the energy change that occurs when an electron is added to a neutral atom in the gas phase. Atoms with high electron affinity tend to gain electrons more readily, while those with low electron affinity are less likely to do so.
Analyzing the Options
- Nitrogen (N):
- Has a half-filled p-orbital configuration, making it relatively stable.
- Its electron affinity is negative, indicating it does not favorably accept an electron.
- Oxygen (O):
- Has a higher electron affinity than nitrogen due to its increased nuclear charge.
- Accepting an electron leads to a more stable configuration, resulting in a significant release of energy.
- Argon (Ar):
- A noble gas with a completely filled outer shell (octet).
- This stable configuration means it has very little tendency to gain an electron, resulting in a low or negligible electron affinity.
- Boron (B):
- While not as stable as nitrogen, it can accept an electron, albeit less favorably than oxygen.
- It has a positive electron affinity, indicating it can gain an electron but with less energy release than oxygen.
Conclusion: Why Argon Has the Lowest Electron Affinity
- Noble Gas Stability: Argon, being a noble gas, has a fully filled valence shell. This configuration inherently makes it very stable and not inclined to accept additional electrons.
- Comparative Analysis: Among the options given, argon has the least tendency to gain an electron, resulting in the lowest electron affinity.
Thus, the correct answer is option C: Argon.
Electron affinity refers to the energy change that occurs when an electron is added to a neutral atom in the gas phase. Atoms with high electron affinity tend to gain electrons more readily, while those with low electron affinity are less likely to do so.
Analyzing the Options
- Nitrogen (N):
- Has a half-filled p-orbital configuration, making it relatively stable.
- Its electron affinity is negative, indicating it does not favorably accept an electron.
- Oxygen (O):
- Has a higher electron affinity than nitrogen due to its increased nuclear charge.
- Accepting an electron leads to a more stable configuration, resulting in a significant release of energy.
- Argon (Ar):
- A noble gas with a completely filled outer shell (octet).
- This stable configuration means it has very little tendency to gain an electron, resulting in a low or negligible electron affinity.
- Boron (B):
- While not as stable as nitrogen, it can accept an electron, albeit less favorably than oxygen.
- It has a positive electron affinity, indicating it can gain an electron but with less energy release than oxygen.
Conclusion: Why Argon Has the Lowest Electron Affinity
- Noble Gas Stability: Argon, being a noble gas, has a fully filled valence shell. This configuration inherently makes it very stable and not inclined to accept additional electrons.
- Comparative Analysis: Among the options given, argon has the least tendency to gain an electron, resulting in the lowest electron affinity.
Thus, the correct answer is option C: Argon.
Which is correct increasing order of their tendency of the given elements to form M3- ion?- a)Bi > Sb > As > P > N
- b)Bi < Sb < As < P < N
- c)N < P < Sb < Bi < As
- d)Bi > Sb ~ N ~ P > As
Correct answer is option 'B'. Can you explain this answer?
Which is correct increasing order of their tendency of the given elements to form M3- ion?
a)
Bi > Sb > As > P > N
b)
Bi < Sb < As < P < N
c)
N < P < Sb < Bi < As
d)
Bi > Sb ~ N ~ P > As
|
|
Gaurav Kumar answered |
On moving down the group, the stability of-3 oxidation state decreases. This is due to the following reasons (i) On descending a group the size of the atom or ion increases. As a result, attraction of the nucleus per newly added electron decreases (ii) A large anion cannot fit easily into lattice ofa small cation, (iii) As the negative charge on the ion increases, it becomes more and more susceptible to polarisation.
According to the law of conservation of momentum,- a)the momentum of an isolated system of interacting particles is conserved
- b)the total momentum of an interacting system of particles and fields is conserved
- c)the total momentum of an isolated system of interacting particles is conserved.
- d)the momentum of an interacting system of particles and fields is conserved
Correct answer is option 'C'. Can you explain this answer?
According to the law of conservation of momentum,
a)
the momentum of an isolated system of interacting particles is conserved
b)
the total momentum of an interacting system of particles and fields is conserved
c)
the total momentum of an isolated system of interacting particles is conserved.
d)
the momentum of an interacting system of particles and fields is conserved

|
Arya Reddy answered |
Explanation:when no external forces acts on a system of several interacting particles, then the total momentum is conserved.
Why is the electron gain enthalpy of O or F less than that of S or Cl?- a)O and F are more electronegative than S and Cl
- b)When an electron is added to O or F, it goes to a smaller (n = 2) level and suffers more repulsion than the electron in S or Cl in larger level (n = 3)
- c)Adding an electron to 3p-orbital leads to more repulsion than 2p-orbital
- d)Electroh gain enthalpy depends upon the electron affinity of the atom
Correct answer is option 'B'. Can you explain this answer?
Why is the electron gain enthalpy of O or F less than that of S or Cl?
a)
O and F are more electronegative than S and Cl
b)
When an electron is added to O or F, it goes to a smaller (n = 2) level and suffers more repulsion than the electron in S or Cl in larger level (n = 3)
c)
Adding an electron to 3p-orbital leads to more repulsion than 2p-orbital
d)
Electroh gain enthalpy depends upon the electron affinity of the atom
|
|
Anjali Sharma answered |
There is more repulsion for the incoming electron when the size of atom is smaller.
The first element of the groups 1 and 2 are different from other members of the respective groups. Their behaviour is more similar to the second element of the following groups. What is this relationship known as?- a)Anomalous relationship
- b)Periodic relationship
- c)Diagonal relationship
- d)Chemical relationship
Correct answer is option 'C'. Can you explain this answer?
The first element of the groups 1 and 2 are different from other members of the respective groups. Their behaviour is more similar to the second element of the following groups. What is this relationship known as?
a)
Anomalous relationship
b)
Periodic relationship
c)
Diagonal relationship
d)
Chemical relationship
|
|
Priya Menon answered |
The relation between 1st element of a group and 2nd element of the next group is called diagonal relationship.
There are many elements in the periodic table which exhibit variable valency. This is a particular characteristic of- a)Representative elements
- b)Transition elements
- c)Noble gases
- d)Non-metals
Correct answer is option 'B'. Can you explain this answer?
There are many elements in the periodic table which exhibit variable valency. This is a particular characteristic of
a)
Representative elements
b)
Transition elements
c)
Noble gases
d)
Non-metals
|
|
Arya Bose answered |
Understanding Variable Valency
Variable valency refers to the ability of an element to exhibit different oxidation states or valencies. This characteristic is most commonly associated with transition elements.
Why Transition Elements Exhibit Variable Valency
- Presence of d-orbitals: Transition metals have partially filled d-orbitals, which allows them to lose different numbers of electrons.
- Multiple oxidation states: They can exhibit multiple oxidation states due to their ability to lose different numbers of electrons from both s and d subshells.
- Formation of complex ions: Transition elements can form complex ions, which can further allow them to display variable valencies depending on the ligands attached.
Examples of Transition Elements with Variable Valency
- Iron (Fe): Exhibits +2 and +3 states.
- Copper (Cu): Shows +1 and +2 states.
- Manganese (Mn): Can have oxidation states ranging from +2 to +7.
Distinction from Other Elements
- Representative Elements: Typically have fixed valencies based on their group number and do not exhibit the same level of variability.
- Noble Gases: Generally do not form compounds and have a complete valence shell, hence no variable valency.
- Non-metals: While some non-metals can have multiple oxidation states (like sulfur), they do not exhibit the extensive variability seen in transition metals.
In conclusion, the transition elements are unique in their ability to showcase variable valency due to their electronic configuration and involvement in complex formation. This property is essential in various chemical reactions and applications, making them a significant focus in chemistry.
Variable valency refers to the ability of an element to exhibit different oxidation states or valencies. This characteristic is most commonly associated with transition elements.
Why Transition Elements Exhibit Variable Valency
- Presence of d-orbitals: Transition metals have partially filled d-orbitals, which allows them to lose different numbers of electrons.
- Multiple oxidation states: They can exhibit multiple oxidation states due to their ability to lose different numbers of electrons from both s and d subshells.
- Formation of complex ions: Transition elements can form complex ions, which can further allow them to display variable valencies depending on the ligands attached.
Examples of Transition Elements with Variable Valency
- Iron (Fe): Exhibits +2 and +3 states.
- Copper (Cu): Shows +1 and +2 states.
- Manganese (Mn): Can have oxidation states ranging from +2 to +7.
Distinction from Other Elements
- Representative Elements: Typically have fixed valencies based on their group number and do not exhibit the same level of variability.
- Noble Gases: Generally do not form compounds and have a complete valence shell, hence no variable valency.
- Non-metals: While some non-metals can have multiple oxidation states (like sulfur), they do not exhibit the extensive variability seen in transition metals.
In conclusion, the transition elements are unique in their ability to showcase variable valency due to their electronic configuration and involvement in complex formation. This property is essential in various chemical reactions and applications, making them a significant focus in chemistry.
Which of the following are correct?
(i)Diploblastic:Poriferans, Coelenterates
(ii)Triploblastic:Platyheliminthes to Chorodates
(iii)Acoelomate:Poriferans,Coelenterates,Platyhelminthes
(iv)Pseudocoelomate:Aschelminthes /Roundworms
(v)Eucoelomate:Annelids to Chordates
- a)(iv) and (v) are correct
- b)(i), (ii), (iii) ,(iv) and (v) are correct
- c)(i), (ii) and (v) are correct
- d)(iii) and (v) are correct
Correct answer is option 'B'. Can you explain this answer?
Which of the following are correct?
(i)Diploblastic:Poriferans, Coelenterates
(ii)Triploblastic:Platyheliminthes to Chorodates
(iii)Acoelomate:Poriferans,Coelenterates,Platyhelminthes
(iv)Pseudocoelomate:Aschelminthes /Roundworms
(v)Eucoelomate:Annelids to Chordates
(i)Diploblastic:Poriferans, Coelenterates
(ii)Triploblastic:Platyheliminthes to Chorodates
(iii)Acoelomate:Poriferans,Coelenterates,Platyhelminthes
(iv)Pseudocoelomate:Aschelminthes /Roundworms
(v)Eucoelomate:Annelids to Chordates
a)
(iv) and (v) are correct
b)
(i), (ii), (iii) ,(iv) and (v) are correct
c)
(i), (ii) and (v) are correct
d)
(iii) and (v) are correct
|
|
Anjali Sharma answered |
(i) Diploblastic - Poriferans, Coelenterates
(ii) Triploblastic - Platyhelminthes to Chordates
(iii) Acoelomate - Poriferans, Coelenterates, Platyhelminthes
(iv) Pseudocoelomate - Aschelminthes/Roundworms
(v) Eucoelomate - Annelids to Chordates
So, the correct answer is '(i), (ii), (iii), (iv) and (v)'.
The correct order of acidic character of oxides in third period of periodic table is
- a)SiO2 < P4O10 < SO3 < CI2O7
- b)Cl2O7 < SO3 < P4O10 < SiO2
- c)SO3 < CI2O2 < P4O10 < SiO2
- d)SiO2 < CI2O7 < P4O10 < SO3
Correct answer is option 'A'. Can you explain this answer?
The correct order of acidic character of oxides in third period of periodic table is
a)
SiO2 < P4O10 < SO3 < CI2O7
b)
Cl2O7 < SO3 < P4O10 < SiO2
c)
SO3 < CI2O2 < P4O10 < SiO2
d)
SiO2 < CI2O7 < P4O10 < SO3
|
|
Jyoti Sengupta answered |
Acidic character of oxides increases as we move from left to right in a period.
As we move from left to right, the electronegativity increases. An atom which is highly electronegative has- a)large size
- b)low electron affinity
- c)high ionisation enthalpy
- d)low chemical reactivity
Correct answer is option 'C'. Can you explain this answer?
As we move from left to right, the electronegativity increases. An atom which is highly electronegative has
a)
large size
b)
low electron affinity
c)
high ionisation enthalpy
d)
low chemical reactivity
|
|
Meera Singh answered |
It is difficult to remove an electron from a highly electronegative element.
Which of the following elettients will have highest second ionisation enthalpy?- a)1s22s22p63s2
- b)1s22s22p63s1
- c)1s22s22p63s23p2
- d)1s22s22p63s23p3
Correct answer is option 'B'. Can you explain this answer?
Which of the following elettients will have highest second ionisation enthalpy?
a)
1s22s22p63s2
b)
1s22s22p63s1
c)
1s22s22p63s23p2
d)
1s22s22p63s23p3
|
|
Rithika Basu answered |
Understanding Ionisation Enthalpy
Ionisation enthalpy refers to the amount of energy required to remove an electron from an atom or ion. The second ionisation enthalpy is the energy needed to remove a second electron from a positively charged ion.
Analyzing the Given Options
- Option A: 1s²2s²2p⁶3s²
- It has two electrons in the outermost shell (3s²). Removing one electron results in a stable noble gas configuration (1s²2s²2p⁶). The second ionisation involves removing an electron from a stable configuration, which requires significant energy.
- Option B: 1s²2s²2p⁶3s¹
- This configuration has only one electron in the outermost shell (3s¹). The removal of the first electron will result in a stable noble gas core (1s²2s²2p⁶). The second ionisation requires removing an electron from a stable core, which demands a high amount of energy.
- Option C: 1s²2s²2p⁶3s²3p²
- This atom has electrons in both 3s and 3p. The second ionisation involves removing an electron from a less stable outer shell, hence lower energy than option B.
- Option D: 1s²2s²2p⁶3s²3p³
- Similar to option C, it has a stable configuration but less than option B, as it has more electrons in the outer shell making it easier to remove an electron.
Conclusion
The highest second ionisation enthalpy is found in option B (1s²2s²2p⁶3s¹) because upon removing the first electron, the resulting ion has a stable noble gas configuration. This configuration makes the second ionisation significantly more energy-demanding compared to the other options.
Ionisation enthalpy refers to the amount of energy required to remove an electron from an atom or ion. The second ionisation enthalpy is the energy needed to remove a second electron from a positively charged ion.
Analyzing the Given Options
- Option A: 1s²2s²2p⁶3s²
- It has two electrons in the outermost shell (3s²). Removing one electron results in a stable noble gas configuration (1s²2s²2p⁶). The second ionisation involves removing an electron from a stable configuration, which requires significant energy.
- Option B: 1s²2s²2p⁶3s¹
- This configuration has only one electron in the outermost shell (3s¹). The removal of the first electron will result in a stable noble gas core (1s²2s²2p⁶). The second ionisation requires removing an electron from a stable core, which demands a high amount of energy.
- Option C: 1s²2s²2p⁶3s²3p²
- This atom has electrons in both 3s and 3p. The second ionisation involves removing an electron from a less stable outer shell, hence lower energy than option B.
- Option D: 1s²2s²2p⁶3s²3p³
- Similar to option C, it has a stable configuration but less than option B, as it has more electrons in the outer shell making it easier to remove an electron.
Conclusion
The highest second ionisation enthalpy is found in option B (1s²2s²2p⁶3s¹) because upon removing the first electron, the resulting ion has a stable noble gas configuration. This configuration makes the second ionisation significantly more energy-demanding compared to the other options.
Which of the following statements regarding the variation of atomic radii in the periodic table is not true?- a)In a group, there is continuous increase in size with increase in atomic number
- b)In 4f-series, there is a continuous decrease in size with increase in atomic number
- c)The size of inert gases is larger than halogens
- d)In 3rd period, the size of atoms increases with increase in atomic number
Correct answer is option 'D'. Can you explain this answer?
Which of the following statements regarding the variation of atomic radii in the periodic table is not true?
a)
In a group, there is continuous increase in size with increase in atomic number
b)
In 4f-series, there is a continuous decrease in size with increase in atomic number
c)
The size of inert gases is larger than halogens
d)
In 3rd period, the size of atoms increases with increase in atomic number
|
|
Shanaya Sengupta answered |
Explanation:
In the periodic table, the atomic radius refers to the size of an atom. It is defined as the distance between the nucleus and the outermost electron shell of an atom. The atomic radius varies across the periodic table due to the distribution of electrons and the effective nuclear charge experienced by the outermost electrons.
Statement (a): In a group, there is continuous increase in size with increase in atomic number
This statement is true. In a group, elements have the same number of valence electrons and similar electron configurations. As we move down a group, the number of electron shells increases, resulting in an increase in atomic size. This is due to the shielding effect of inner electron shells, which reduces the attraction between the nucleus and the outermost electrons.
Statement (b): In 4f-series, there is a continuous decrease in size with increase in atomic number
This statement is true. The 4f-series consists of the lanthanides, which are located in the f-block of the periodic table. As we move across the lanthanide series, the atomic number increases, resulting in a decrease in atomic size. This is because the increasing number of protons in the nucleus exerts a stronger attraction on the electrons, leading to a contraction in atomic radius.
Statement (c): The size of inert gases is larger than halogens
This statement is true. Inert gases, also known as noble gases, have completely filled electron shells. They are located in Group 18 of the periodic table. The halogens, on the other hand, are located in Group 17. Inert gases have larger atomic radii compared to halogens because inert gases have more electron shells and experience less effective nuclear charge due to greater electron-electron repulsion.
Statement (d): In 3rd period, the size of atoms increases with increase in atomic number
This statement is not true. In the 3rd period, the atomic size does not strictly increase with atomic number. The trend is not continuous due to the difference in electron configurations. While the atomic size generally increases from left to right across a period, there are exceptions due to variations in the effective nuclear charge and electron-electron repulsion. For example, the atomic radius of sodium (Na) is larger than that of magnesium (Mg) in the 3rd period.
Therefore, the correct answer is option D - In the 3rd period, the size of atoms increases with increase in atomic number.
In the periodic table, the atomic radius refers to the size of an atom. It is defined as the distance between the nucleus and the outermost electron shell of an atom. The atomic radius varies across the periodic table due to the distribution of electrons and the effective nuclear charge experienced by the outermost electrons.
Statement (a): In a group, there is continuous increase in size with increase in atomic number
This statement is true. In a group, elements have the same number of valence electrons and similar electron configurations. As we move down a group, the number of electron shells increases, resulting in an increase in atomic size. This is due to the shielding effect of inner electron shells, which reduces the attraction between the nucleus and the outermost electrons.
Statement (b): In 4f-series, there is a continuous decrease in size with increase in atomic number
This statement is true. The 4f-series consists of the lanthanides, which are located in the f-block of the periodic table. As we move across the lanthanide series, the atomic number increases, resulting in a decrease in atomic size. This is because the increasing number of protons in the nucleus exerts a stronger attraction on the electrons, leading to a contraction in atomic radius.
Statement (c): The size of inert gases is larger than halogens
This statement is true. Inert gases, also known as noble gases, have completely filled electron shells. They are located in Group 18 of the periodic table. The halogens, on the other hand, are located in Group 17. Inert gases have larger atomic radii compared to halogens because inert gases have more electron shells and experience less effective nuclear charge due to greater electron-electron repulsion.
Statement (d): In 3rd period, the size of atoms increases with increase in atomic number
This statement is not true. In the 3rd period, the atomic size does not strictly increase with atomic number. The trend is not continuous due to the difference in electron configurations. While the atomic size generally increases from left to right across a period, there are exceptions due to variations in the effective nuclear charge and electron-electron repulsion. For example, the atomic radius of sodium (Na) is larger than that of magnesium (Mg) in the 3rd period.
Therefore, the correct answer is option D - In the 3rd period, the size of atoms increases with increase in atomic number.
A stationary ball weighing 0.25kg acquires a speed of 10m/s when hit by a hockey stick. The impulse imparted to the ball is
- a)1.5 Ns
- b)0.5 Ns
- c)2.5 Ns
- d)2.0 Ns
Correct answer is option 'C'. Can you explain this answer?
A stationary ball weighing 0.25kg acquires a speed of 10m/s when hit by a hockey stick. The impulse imparted to the ball is
a)
1.5 Ns
b)
0.5 Ns
c)
2.5 Ns
d)
2.0 Ns
|
|
Nandini Iyer answered |
Initial momentum of the ball,
Pi = 0
Final momentum of the ball,
Pf = mv
⇒ 0.25×10 = 2.5 kg m/s
Impulse imparted on the ball,
I = Pf − Pi
⇒ I = 2.5 − 0 = 2.5 N s
Pi = 0
Final momentum of the ball,
Pf = mv
⇒ 0.25×10 = 2.5 kg m/s
Impulse imparted on the ball,
I = Pf − Pi
⇒ I = 2.5 − 0 = 2.5 N s
Friction is- a)perpendicular to contact surface and aids motion
- b)parallel to contact surface and aids motion
- c)perpendicular to contact surface and opposes i motion
- d)parallel to contact surface and opposes the relative motion
Correct answer is option 'D'. Can you explain this answer?
Friction is
a)
perpendicular to contact surface and aids motion
b)
parallel to contact surface and aids motion
c)
perpendicular to contact surface and opposes i motion
d)
parallel to contact surface and opposes the relative motion

|
Navya Sengupta answered |
Explanation:Whenever a body of mass m moves or tends to move over the surface of another body, then the opposite force acts parallel to the surface contact which opposes the relative motion.
A man of mass 70 kg stands on a weighing scale in a lift which is moving upwards with a uniform acceleration of 5 m s−2 what would be the reading on the scale?- a)125 kg
- b)105 kg
- c)95 kg Hz
- d)115 kg
Correct answer is option 'B'. Can you explain this answer?
A man of mass 70 kg stands on a weighing scale in a lift which is moving upwards with a uniform acceleration of 5 m s−2 what would be the reading on the scale?
a)
125 kg
b)
105 kg
c)
95 kg Hz
d)
115 kg
|
|
Preeti Iyer answered |
Mass of the man, m = 70 kg
Acceleration, a = 5 m/s2 upward
Using Newton’s second law of motion, we can write the equation of motion as:
R – mg = ma
R = m(g + a)
= 70 (10 + 5) = 70 x 15
= 1050 N
∴ Reading on the weighing scale = 1050 / g = 1050 / 10 = 105 kg
Chapter doubts & questions for June Week 3 - Weekly Tests for NEET Preparation 2025 is part of NEET exam preparation. The chapters have been prepared according to the NEET exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for NEET 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of June Week 3 - Weekly Tests for NEET Preparation in English & Hindi are available as part of NEET exam.
Download more important topics, notes, lectures and mock test series for NEET Exam by signing up for free.
Related NEET Content

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up
within 7 days!
within 7 days!
Takes less than 10 seconds to signup









