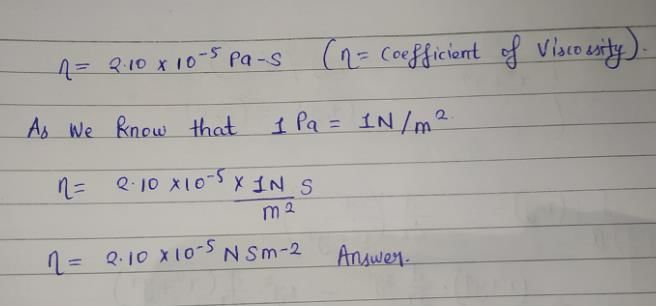All Exams >
JEE >
6 Months Preparation for JEE >
All Questions
All questions of Liquid State for JEE Exam
Direction (Q. Nos. 1-6) This section contains 6 multiple choice questions. Each question has four
choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.Q. Which pair of molecules has the strongest dipole-dipole interactions?- a)NH3 and CH4
- b)CH4 and CH4
- c)CO3 and CO2
- d)NH3 and NH3
Correct answer is 'D'. Can you explain this answer?
Direction (Q. Nos. 1-6) This section contains 6 multiple choice questions. Each question has four
choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Q. Which pair of molecules has the strongest dipole-dipole interactions?
a)
NH3 and CH4
b)
CH4 and CH4
c)
CO3 and CO2
d)
NH3 and NH3
|
|
Naina Bansal answered |
NH3 and NH3. Both polar, asymmetric molecules. CH4 is nonpolar. CO2 is symetrical.
Select the correct statement(s).- a)Cohesive forces are the intermolecular forces between like molecules and adhesive forces are between unlike molecules
- b)A drop maintains its shape if cohesive forces are stronger than adhesive forces
- c)If cohesive forces are weak compared to adhesive forces, drop collapses and spreads into film
- d)Cohesive forces in mercury, consiste of metallic bonds between atoms, are strong; thus it does not wet glass
Correct answer is option 'A,B,C,D'. Can you explain this answer?
Select the correct statement(s).
a)
Cohesive forces are the intermolecular forces between like molecules and adhesive forces are between unlike molecules
b)
A drop maintains its shape if cohesive forces are stronger than adhesive forces
c)
If cohesive forces are weak compared to adhesive forces, drop collapses and spreads into film
d)
Cohesive forces in mercury, consiste of metallic bonds between atoms, are strong; thus it does not wet glass
|
|
Om Desai answered |
a) True, cohesive forces are intermolecular forces between like molecules and adhesive forces between unlike molecules.
b) True, only due to cohesive force, all the molecules of droplets(which are like molecule) are attracted towards each other to form drop
c) True, if the cohesive force becomes weak as to adhesive forces, then there will be no force to bind water molecules and the drop will collapse and spread into film.
d) True, It's only due to cohesive force that mercury doesn’t wet the glass.
b) True, only due to cohesive force, all the molecules of droplets(which are like molecule) are attracted towards each other to form drop
c) True, if the cohesive force becomes weak as to adhesive forces, then there will be no force to bind water molecules and the drop will collapse and spread into film.
d) True, It's only due to cohesive force that mercury doesn’t wet the glass.
Stronger intermolecular forces result in higher boiling point. Strength of London forces increases with number of electrons in the molecule. Boiling point of  Q. Based on the boiling points, predominant force which gives variation of boiling point of HCI < HBr < HI is
Q. Based on the boiling points, predominant force which gives variation of boiling point of HCI < HBr < HI is- a)London interaction
- b)dipole-dipole interaction
- c)hydrogen bonding
- d)dipole-induced dipole interaction
Correct answer is option 'A'. Can you explain this answer?
Stronger intermolecular forces result in higher boiling point. Strength of London forces increases with number of electrons in the molecule. Boiling point of
Q. Based on the boiling points, predominant force which gives variation of boiling point of HCI < HBr < HI is
a)
London interaction
b)
dipole-dipole interaction
c)
hydrogen bonding
d)
dipole-induced dipole interaction
|
|
Rajeev Saxena answered |
(a) HF - Hydrogen bonding HCl,HBr,HI→ Dipole-dipole interaction, London-dispersion force.
Vapour pressure of mixture of 1 mole volatile liquid A and 1 mole volatile liquid B is 350 mm Hg at 50°C. On adding 2 moles of A Into mixture, vapour pressure increases by 25 mm Hg. Thus, vapour pressure of pure components are (in mm Hg)
- a)a
- b)b
- c)c
- d)d
Correct answer is option 'A'. Can you explain this answer?
Vapour pressure of mixture of 1 mole volatile liquid A and 1 mole volatile liquid B is 350 mm Hg at 50°C. On adding 2 moles of A Into mixture, vapour pressure increases by 25 mm Hg. Thus, vapour pressure of pure components are (in mm Hg)
a)
a
b)
b
c)
c
d)
d
|
|
Krishna Iyer answered |
We have from Raoult’s law;-
Pmixture = xaPa+xbPb
350 = 1/2×Pa+1/2×Pb
Pa+Pb = 700
And 375 = 3/4Pa+1/4pb
On solving both equations, we get Pa = 400 and Pb = 300
Pmixture = xaPa+xbPb
350 = 1/2×Pa+1/2×Pb
Pa+Pb = 700
And 375 = 3/4Pa+1/4pb
On solving both equations, we get Pa = 400 and Pb = 300
In comparing gases with liquids , gases have ........ compressibility and...........density.- a)greater, smalle
- b)greater, greater
- c)smaller, smaller
- d)smaller, greater
Correct answer is option 'A'. Can you explain this answer?
In comparing gases with liquids , gases have ........ compressibility and...........density.
a)
greater, smalle
b)
greater, greater
c)
smaller, smaller
d)
smaller, greater
|
|
Neha Patel answered |
In a gas, the distance between molecules, whether monatomic or polyatomic, is very large compared with the size of the molecules; thus gases have a low density and are highly compressible.Density: The molecules of a liquid are packed relatively close together. Consequently, liquids are much denser than gases.
Surface tension of water is 73 dynes cm -1 at 20° C. If surface area is increased by 0.10 m2, work done is- a)7.3 erg
- b)7.3 x 104 erg
- c)7.3 J
- d)0.73 J
Correct answer is option 'B'. Can you explain this answer?
Surface tension of water is 73 dynes cm -1 at 20° C. If surface area is increased by 0.10 m2, work done is
a)
7.3 erg
b)
7.3 x 104 erg
c)
7.3 J
d)
0.73 J
|
|
Lavanya Menon answered |
Work done = surface tension×Change in area
Surface tension = 73 dyne cm-1 or 73 x 10-3 N/m
Area = 0.10 m2
So, work done = 73×10-3 x 0.10
= 73 x 10-4 J or 73×10-4 x 107 erg
= 7.3×104 erg
Surface tension = 73 dyne cm-1 or 73 x 10-3 N/m
Area = 0.10 m2
So, work done = 73×10-3 x 0.10
= 73 x 10-4 J or 73×10-4 x 107 erg
= 7.3×104 erg
Hydrogen bonding reduces the quality of water molecules to
- a)repel
- b)attract
- c)compactly arrange
- d)slide over each other
Correct answer is option 'D'. Can you explain this answer?
Hydrogen bonding reduces the quality of water molecules to
a)
repel
b)
attract
c)
compactly arrange
d)
slide over each other
|
|
Shreya Gupta answered |
Hydrogen bonding is a type of attractive force that occurs between molecules when a hydrogen atom is covalently bonded to a highly electronegative atom, such as oxygen, nitrogen, or fluorine. In water molecules, hydrogen bonding occurs between the positively charged hydrogen atoms of one water molecule and the negatively charged oxygen atoms of another water molecule. These hydrogen bonds cause the water molecules to attract each other and stick together, which gives water many of its unique properties, such as its high surface tension and its ability to act as a solvent. The hydrogen bonds do not cause the water molecules to repel each other or to compactly arrange, but they do make it more difficult for the molecules to slide over each other, which contributes to the high viscosity of water.
At a given temperature, total vapour pressure (in torr) of a mixture of volatile components A and B is g iven by ptotal = 120 - 75 x B.Hence, vapour pressure of pure A and B respectively are- a)120,75
- b)120,95
- c)120,45
- d)75,45
Correct answer is option 'C'. Can you explain this answer?
At a given temperature, total vapour pressure (in torr) of a mixture of volatile components A and B is g iven by ptotal = 120 - 75 x B.
Hence, vapour pressure of pure A and B respectively are
a)
120,75
b)
120,95
c)
120,45
d)
75,45
|
|
Hansa Sharma answered |
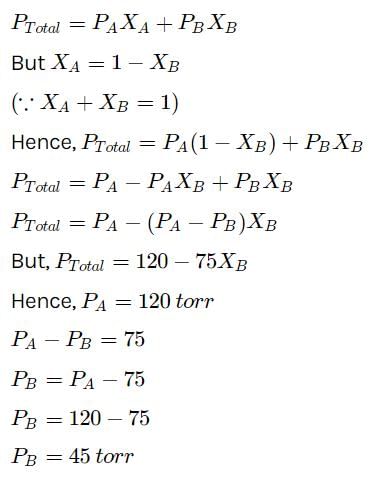
Which of the following properties of water can be used to explain the spherical shape of rain droplets?- a)Viscosity
- b)Surface tension
- c)Critical phenomenon
- d)Vapour pressure
Correct answer is option 'B'. Can you explain this answer?
Which of the following properties of water can be used to explain the spherical shape of rain droplets?
a)
Viscosity
b)
Surface tension
c)
Critical phenomenon
d)
Vapour pressure
|
|
Naina Bansal answered |
A simple way to form a drop is to allow liquid to flow slowly from the lower end of a vertical tube of small diameter. The surface tension of the liquid causes the liquid to hang from the tube, forming a pendant. When the drop exceeds a certain size it is no longer stable and detaches itself. The falling liquid is also a drop held together by surface tension.
As the temperature is raised from 20°C to 40°C, the average kinetic energy of neon atoms changes by a factor of which of the following ? [AIEEE-2004]- a)
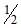
- b)
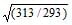
- c)
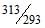
- d)2
Correct answer is option 'C'. Can you explain this answer?
As the temperature is raised from 20°C to 40°C, the average kinetic energy of neon atoms changes by a factor of which of the following ? [AIEEE-2004]
a)
b)
c)
d)
2
|
|
Shreya Gupta answered |
Average kinetic energy ∝ Temperature in Kelvin
(KE40) / (KE20) = (3/2 nR * 313)/(3/2 nR*293)
KE40 / KE20 = T2/T1 = 40 + 273 / 20 + 273 = 313/293
Direction (Q. Nos. 12-15) This section contains a paragraph, wach describing theory, experiments, data etc. three Questions related to paragraph have been given.Each question have only one correct answer among the four given options (a),(b),(c),(d).Passage IFor a given liquid at a given temperature vapour pressure is given by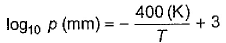 Q. Thus, vapour pressure of the liquid at 400 K is
Q. Thus, vapour pressure of the liquid at 400 K is- a)2 mm
- b)100 mm
- c)103 mm
- d)20 mm
Correct answer is option 'B'. Can you explain this answer?
Direction (Q. Nos. 12-15) This section contains a paragraph, wach describing theory, experiments, data etc. three Questions related to paragraph have been given.Each question have only one correct answer among the four given options (a),(b),(c),(d).
Passage I
For a given liquid at a given temperature vapour pressure is given by
Q. Thus, vapour pressure of the liquid at 400 K is
a)
2 mm
b)
100 mm
c)
103 mm
d)
20 mm
|
|
Gaurav Kumar answered |
Substitute T equal to 400 K in RHS and solve the log.
log(base 10)p = 2
⇒ 10² = p
⇒ p = 100 mm
log(base 10)p = 2
⇒ 10² = p
⇒ p = 100 mm
The correct value of R is - [aieee-2002]- a)R = 0.082 litre-atm
- b)R = 8.314 × 107 erg K-1 mol-1
- c)R = 2 k-1 mol-1
- d) None
Correct answer is option 'B'. Can you explain this answer?
The correct value of R is - [aieee-2002]
a)
R = 0.082 litre-atm
b)
R = 8.314 × 107 erg K-1 mol-1
c)
R = 2 k-1 mol-1
d)
None
|
|
Lavanya Menon answered |
The different values of R are as follow:-
8.314 J mol-1 K-1, 8.314×107 erg mol-1 K-1, 0.0821 atm-lit mol-1 K-1 or 2 cal mol-1 K-1
8.314 J mol-1 K-1, 8.314×107 erg mol-1 K-1, 0.0821 atm-lit mol-1 K-1 or 2 cal mol-1 K-1
When r, P and M represent rate of diffusion, pressure and molecular mass, respectively, then the ratio of the rates of diffusion (rA/rB) of two gases A and B, is given as - [AIEEE - 2011]- a)(PA/PB) (MB/MA)1/2
- b)(PA/PB)1/2 (MB/MA)
- c)(PA/PB) (MA/MB)1/2
- d)(PA/PB)1/2 (MA/MB)
Correct answer is option 'A'. Can you explain this answer?
When r, P and M represent rate of diffusion, pressure and molecular mass, respectively, then the ratio of the rates of diffusion (rA/rB) of two gases A and B, is given as -
[AIEEE - 2011]
a)
(PA/PB) (MB/MA)1/2
b)
(PA/PB)1/2 (MB/MA)
c)
(PA/PB) (MA/MB)1/2
d)
(PA/PB)1/2 (MA/MB)
|
|
Raghav Bansal answered |
The correct answer is option A
Rate of diffusion: r α p

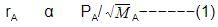
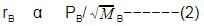
rA/rB = pA/pB.(MB/MA)1/2
Hence, pBpA (MB/MA)1/2 is the answer.
Rate of diffusion: r α p

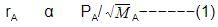
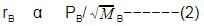
rA/rB = pA/pB.(MB/MA)1/2
Hence, pBpA (MB/MA)1/2 is the answer.
Intermolecular forces can be out of the following.- a)van der Waais' forces
- b)Electrostatic forces existing between two oppositely charged ions
- c)Covalent bond between two like atoms
- d)Gravitational force
Correct answer is option 'A'. Can you explain this answer?
Intermolecular forces can be out of the following.
a)
van der Waais' forces
b)
Electrostatic forces existing between two oppositely charged ions
c)
Covalent bond between two like atoms
d)
Gravitational force
|
|
Shreya Gupta answered |
In molecular physics, the van der Waals forces, named after Dutch scientist Johannes Diderik van der Waals, are distance-dependent interactions between atoms or molecules. Unlike ionic or covalent bonds, these attractions are not a result of any chemical electronic bond, and they are comparatively weak and more susceptible to being perturbed. Van der Waals forces quickly vanish at longer distances between interacting molecules.
Van der Waals forces play a fundamental role in fields as diverse as supramolecular chemistry, structural biology, polymer science, nanotechnology, surface science, and condensed matter physics. Van der Waals forces also define many properties of organic compounds and molecular solids, including their solubility in polar and non-polar media.
Direction (Q. Nos. 16 and 17) This section contains 2 questions. when worked out will result in an integer from 0 to 9 (both inclusive).Q. The vapour pressure of benzene C6H6 at 298 K is 95 torr. After 10.00 g of benzene is injected into a 10.0 L bulb at 298 K, how many grams of benzene remain as liquid?
Correct answer is '5'. Can you explain this answer?
Direction (Q. Nos. 16 and 17) This section contains 2 questions. when worked out will result in an integer from 0 to 9 (both inclusive).
Q. The vapour pressure of benzene C6H6 at 298 K is 95 torr. After 10.00 g of benzene is injected into a 10.0 L bulb at 298 K, how many grams of benzene remain as liquid?
|
|
Sanchita Chakraborty answered |
Question Analysis
The question provides the initial conditions of a system (vapour pressure of benzene at 298 K) and asks for the final state of the system (grams of benzene remaining as liquid after injection into a bulb). To solve this problem, we need to apply the concept of vapour pressure and use the ideal gas law.
Solution
Step 1: Calculate the number of moles of benzene
Given:
- Mass of benzene (m) = 10.00 g
- Molar mass of benzene (M) = 78.11 g/mol
Using the formula:
Number of moles (n) = mass / molar mass
Substituting the given values:
n = 10.00 g / 78.11 g/mol
n ≈ 0.128 mol
Step 2: Calculate the initial number of moles of benzene in the vapor phase
Given:
- Total volume of the bulb (V) = 10.0 L
- Vapour pressure of benzene (P) = 95 torr
Using the ideal gas law:
PV = nRT
Rearranging the formula:
n = PV / RT
Substituting the given values:
n = (95 torr) * (10.0 L) / (0.0821 L·atm/mol·K) * (298 K)
n ≈ 3.84 mol
Step 3: Calculate the number of moles of benzene in the liquid phase
Given:
- Initial number of moles of benzene = 0.128 mol
- Number of moles of benzene in the vapor phase = 3.84 mol
Using the law of conservation of mass:
Number of moles of benzene in the liquid phase = Initial number of moles - Number of moles in the vapor phase
Substituting the given values:
Number of moles of benzene in the liquid phase = 0.128 mol - 3.84 mol
Number of moles of benzene in the liquid phase ≈ -3.712 mol
Step 4: Calculate the mass of benzene remaining as liquid
Given:
- Molar mass of benzene (M) = 78.11 g/mol
Using the formula:
Mass of benzene remaining as liquid = Number of moles of benzene in the liquid phase * Molar mass of benzene
Substituting the given values:
Mass of benzene remaining as liquid = -3.712 mol * 78.11 g/mol
Mass of benzene remaining as liquid ≈ -289.57 g
Since mass cannot be negative, the negative sign indicates an error in the calculations.
Step 5: Identifying the error and correcting it
The error in the calculations is likely due to the assumption that all the benzene injected into the bulb vaporizes. However, in reality, only a fraction of the benzene will vaporize based on its vapour pressure.
To correct the error, we need to determine the fraction of benzene that vaporizes and subtract it from the initial mass of benzene.
Step 6: Calculate the fraction of benz
Van der waals forces include the following except
- a)London forces
- b)dipole - dipole forces
- c)dipole- include dipole forces
- d)chemical bonding forces
Correct answer is option 'D'. Can you explain this answer?
Van der waals forces include the following except
a)
London forces
b)
dipole - dipole forces
c)
dipole- include dipole forces
d)
chemical bonding forces
|
|
Om Desai answered |
Chemical bonding forces are not considered to be part of van der Waals forces. Van der Waals forces include London forces, dipole-dipole forces, and dipole-induced dipole forces.
If 10-4 dm3 of water is introduced into a 1.0 dm3 flask at 300 K, how many moles of water are in in the vapour phase when equilibrium is established ? [aieee-2010](Given : Vapour pressure of H2O at 300 is 3170 pa; R = 8.314 JK-1 mol)- a)1.27 x 10-3 mol
- b)5.56 × 10-3 mol
- c) 1.53 x10-2 mol
- d)4346 × 10-2 mol
Correct answer is option 'A'. Can you explain this answer?
If 10-4 dm3 of water is introduced into a 1.0 dm3 flask at 300 K, how many moles of water are in in the vapour phase when equilibrium is established ? [aieee-2010]
(Given : Vapour pressure of H2O at 300 is 3170 pa; R = 8.314 JK-1 mol)
a)
1.27 x 10-3 mol
b)
5.56 × 10-3 mol
c)
1.53 x10-2 mol
d)
4346 × 10-2 mol
|
|
Raghav Bansal answered |
The volume occupied by water molecules in vapour phase is (1×10−4) dm3, that is approximately (1×10−3) m3.
pvapV = nH2O mol
3170 × 1 × 10−3 = nH2O × 8.314 × 300K
nH2O = 3170 × 1 × 10−3 / 8.314 × 300
= 1.27 × 10−3 mol
pvapV = nH2O mol
3170 × 1 × 10−3 = nH2O × 8.314 × 300K
nH2O = 3170 × 1 × 10−3 / 8.314 × 300
= 1.27 × 10−3 mol
A gaseous hydrocarbon gives upon combustion 0.72 g of water and 3.08 g. of CO2. The empirical formula of the Hydrocarbon is : [Jee(Main) 2013, 3/120]- a) C2H4
- b)C3H4
- c)C6H5
- d)C7H8
Correct answer is option 'D'. Can you explain this answer?
A gaseous hydrocarbon gives upon combustion 0.72 g of water and 3.08 g. of CO2. The empirical formula of the Hydrocarbon is : [Jee(Main) 2013, 3/120]
a)
C2H4
b)
C3H4
c)
C6H5
d)
C7H8
|
|
Naina Bansal answered |
General equation for combustion of hydrocarbon:
CxHy + (x+ y/4)O2 → xCO2 + (y/2)H2O
Number of moles of CO2 produced = 3.08/44 = 0.07
Number of moles of H2O produced = 0.72/18 = 0.04
SO, x / (y/2) = 0.07/0.04 = 7/4
The formula of hydrocarbon is C7H8
Hence, the correct option is D.
The no. of moles per litre in the equation PV = nRT is expressed by - [aieee-2002]- a)
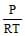
- b)
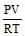
- c)
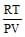
- d)None
Correct answer is option 'A'. Can you explain this answer?
The no. of moles per litre in the equation PV = nRT is expressed by - [aieee-2002]
a)
b)
c)
d)
None

|
Chinmaykumar811 Rout answered |
See qn . given .....per litre..... that means v=1.Then look at options If any option don't contain V that will be answer otherwise select ...none ...Hence option A correct.
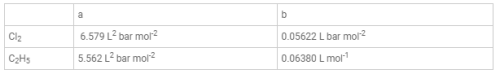
`a' and `b' are Vander Waals' constant for gases. Chlorine is more easily liquefied than ethane because : [aieee-2011]- a) A and b for Cl2 > a and b for C2H6
- b)A and b for Cl2 < a and b for C2H6
- c)A for Cl2 < a for C2H6 but b for Cl2 > b for C2H6
- d)A for Cl2 > a for C2H6 but b for Cl2 < b for C2H6
Correct answer is option 'D'. Can you explain this answer?
`a' and `b' are Vander Waals' constant for gases. Chlorine is more easily liquefied than ethane because : [aieee-2011]
a)
A and b for Cl2 > a and b for C2H6
b)
A and b for Cl2 < a and b for C2H6
c)
A for Cl2 < a for C2H6 but b for Cl2 > b for C2H6
d)
A for Cl2 > a for C2H6 but b for Cl2 < b for C2H6

|
Sanchita Reddy answered |
Vander Waals, constant a is due to force of attraction and b due to the infinite size of molecules. Thus, greater the value a and smaller the value b, larger the liquefaction.

In van der Waals equation of state of the gas law, the constant `b' is a measure of -[AIEEE-2004]- a) Intermolecular repulsions
- b) Intermolecular attraction
- c)Volume occupied by the molecules
- d) Intermolecular collisions per unit volume
Correct answer is option 'C'. Can you explain this answer?
In van der Waals equation of state of the gas law, the constant `b' is a measure of -
[AIEEE-2004]
a)
Intermolecular repulsions
b)
Intermolecular attraction
c)
Volume occupied by the molecules
d)
Intermolecular collisions per unit volume
|
|
Lavanya Menon answered |
The correct answer is Option C.
In van der Waals equation of state of the gas law, the constant b is a measure of the volume occupied by the molecules.
It gives the effective size of the gas molecules. The greater value of b indicates a larger size of the molecules and smaller compressible volume.
It gives the effective size of the gas molecules. The greater value of b indicates a larger size of the molecules and smaller compressible volume.
Dipole-dipole interaction energy between stationary polar molecules is proportional to x and that between rotating molecules is proportional to y. Assume distance between polar molecules as r, then x and y are- a)
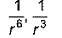
- b)
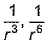
- c)
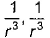
- d)
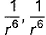
Correct answer is option 'B'. Can you explain this answer?
Dipole-dipole interaction energy between stationary polar molecules is proportional to x and that between rotating molecules is proportional to y. Assume distance between polar molecules as r, then x and y are
a)
b)
c)
d)

|
Ambition Institute answered |
Dipole-dipole interaction energy between stationary polar molecules is proportional to 1/ r3 and that between rotating polar molecules is proportional to 1/ r6 where ‘r’ is the distance between polar molecules
Besides dipole - dipole interaction, polar molecules can interact by London forces also.
Arrange ortho, meta and para-nitrophenols in increasing boiling points- a)para < meta < ortho
- b)ortho < para < meta
- c)ortho = para = meta
- d)ortho < meta < para
Correct answer is option 'D'. Can you explain this answer?
Arrange ortho, meta and para-nitrophenols in increasing boiling points
a)
para < meta < ortho
b)
ortho < para < meta
c)
ortho = para = meta
d)
ortho < meta < para
|
|
Lavanya Menon answered |
Para has max packing efficiency due to its symmetrical structure and it also forms intermolecular H-bonds. Meta derivative has comparatively low packing efficiency but forms intermolecular H-bonds and ortho derivative has the least packing efficiency and does not form intermolecular hydrogen bonds instead it forms intramolecular hydrogen bond which doesn't have any role in increasing the boiling point.
Atom which must be present in hydrogen bonding is- a)hydrogen
- b)sodium
- c)calcium
- d)sulphur
Correct answer is option 'A'. Can you explain this answer?
Atom which must be present in hydrogen bonding is
a)
hydrogen
b)
sodium
c)
calcium
d)
sulphur
|
|
Nandini Patel answered |
A hydrogen bond is the attractive force between the hydrogen attached to an electronegative atom of one molecule and an electronegative atom of a different molecule. Usually the electronegative atom is oxygen, nitrogen, or fluorine, which has a partial negative charge. The hydrogen then has the partial positive charge.
Choose the correct statement with respect to the vapour pressure of a liquid among the following.- a)Increases linearly with increasing temperature
- b)Increases non-linearly with increasing temperature
- c)Decreases linearly with increasing temperature
- d)Decreases non-linearly with increasing temperature
Correct answer is option 'B'. Can you explain this answer?
Choose the correct statement with respect to the vapour pressure of a liquid among the following.
a)
Increases linearly with increasing temperature
b)
Increases non-linearly with increasing temperature
c)
Decreases linearly with increasing temperature
d)
Decreases non-linearly with increasing temperature
|
|
Sarita Yadav answered |
Vapour pressure is defined as the pressure exerted by the vapours above the liquid surface in equilibrium with the liquid at a given temperature. The vapour pressure of a liquid increases non-linearly with increasing temperature.
This is because kinetic energy is the function of temperature which means that as the temperature is increased, more molecules will have greater kinetic energies and thus they can escape from the surface of the liquid to the vapour phase resulting in higher vapour pressure.
This is because kinetic energy is the function of temperature which means that as the temperature is increased, more molecules will have greater kinetic energies and thus they can escape from the surface of the liquid to the vapour phase resulting in higher vapour pressure.
The interaction energy of London force is inversely proportional to sixth power of the distance between two interacting particles but their magnitude depends upon- a)charge of interacting particles
- b)mass of interacting particles
- c)strength of permanent dipoles in the particles
- d)polarisability of interacting particles
Correct answer is option 'D'. Can you explain this answer?
The interaction energy of London force is inversely proportional to sixth power of the distance between two interacting particles but their magnitude depends upon
a)
charge of interacting particles
b)
mass of interacting particles
c)
strength of permanent dipoles in the particles
d)
polarisability of interacting particles
|
|
Rajat Kapoor answered |
The interaction energy of London force is inversely proportional to sixth power of the distance between two interacting particles but their magnitude depends upon. (d) strength of permanent dipoles in the particles.
Based on the following statements I and IS, select the correct answer from the codes given.Statement I Three states of matter are the result of balance between intermolecular forces and thermal energy of the molecules.Statement IIIntermolecular forces tend to keep the molecules together but thermal energy of molecules tends to keep them apart.- a)Both Statement I and Statement II are correct and Statement II is the correct explanation of Statement I
- b)Both Statement I and Statement II are correct and Statement II is not the correct explanation of Statement I
- c)Statement I is correct but Statement il is incorrect
- d)Statement II is correct but Statement I is incorrect
Correct answer is option 'A'. Can you explain this answer?
Based on the following statements I and IS, select the correct answer from the codes given.
Statement I
Three states of matter are the result of balance between intermolecular forces and thermal energy of the molecules.
Statement II
Intermolecular forces tend to keep the molecules together but thermal energy of molecules tends to keep them apart.
a)
Both Statement I and Statement II are correct and Statement II is the correct explanation of Statement I
b)
Both Statement I and Statement II are correct and Statement II is not the correct explanation of Statement I
c)
Statement I is correct but Statement il is incorrect
d)
Statement II is correct but Statement I is incorrect
|
|
Geetika Shah answered |
Thermal energy is the energy of a body arising from motion of its atoms or molecules. It is directly proportional to the temperature of the substance. It is the measure of average kinetic energy of the particles of the matter and is thus responsible for movement of particles. This movement of particles is called thermal motion. We have already learnt that intermolecular forces tend to keep the molecules together but thermal energy of the molecules tends to keep them apart. Three states of matter are the result of balance between intermolecular forces and the thermal energy of the molecules.
Atmospheric pressures recorded in four metro cities are as follows : Q. Based on the above data, order in which water will boil (starting the earliest) in these cities is
Q. Based on the above data, order in which water will boil (starting the earliest) in these cities is- a)I > III > IV > II
- b)II > IV > III > I
- c)I > III > II > IV
- d)IV > II > III > I
Correct answer is option 'C'. Can you explain this answer?
Atmospheric pressures recorded in four metro cities are as follows :
Q. Based on the above data, order in which water will boil (starting the earliest) in these cities is
a)
I > III > IV > II
b)
II > IV > III > I
c)
I > III > II > IV
d)
IV > II > III > I
|
|
Om Desai answered |
For water to boil, it needs to balance the atmospheric pressure exerted on it. It will be easy for water to boil when atmospheric pressure is less. So for the answer, the pressure would be arranged from least pressure to highest pressure and this will be the order of decreasing ease of boiling point.
Direction (Q. Nos. 9) Choice the correct combination of elements and column I and coloumn II are given as option (a), (b), (c) and (d), out of which ONE option is correct.Q. Match the physical properties given in Column I with the corresponding units given in Column II and select the correct answer from the codes given below.

- a)a
- b)b
- c)c
- d)d
Correct answer is option 'A'. Can you explain this answer?
Direction (Q. Nos. 9) Choice the correct combination of elements and column I and coloumn II are given as option (a), (b), (c) and (d), out of which ONE option is correct.
Q. Match the physical properties given in Column I with the corresponding units given in Column II and select the correct answer from the codes given below.
a)
a
b)
b
c)
c
d)
d

|
Ashish Mishra answered |
A is correct.
Arrange the following in increasing order of surface tension.I (water), II (ethanol), III (hexane)- a)I < II < III
- b)II < I < III
- c)Ill < I < II
- d)Ill < II < I
Correct answer is option 'D'. Can you explain this answer?
Arrange the following in increasing order of surface tension.
I (water), II (ethanol), III (hexane)
a)
I < II < III
b)
II < I < III
c)
Ill < I < II
d)
Ill < II < I
|
|
Pranavi Chopra answered |
Understanding Surface Tension
Surface tension is a physical property that describes the cohesive forces acting at the surface of a liquid. It is influenced by the type of molecules present and their interactions.
Comparison of Liquids
1. Water (I)
- High surface tension due to strong hydrogen bonding.
- Molecules are tightly held together, creating a robust surface.
2. Ethanol (II)
- Lower surface tension than water.
- Hydrogen bonds are present, but ethanol has a relatively weaker intermolecular force due to its smaller size compared to water.
3. Hexane (III)
- Lowest surface tension among the three.
- Non-polar molecules result in weaker van der Waals forces, leading to a less cohesive surface.
Order of Surface Tension
- Based on the strength of intermolecular forces:
- Hexane (III) has the weakest intermolecular forces.
- Ethanol (II) has moderate forces.
- Water (I) exhibits the strongest intermolecular forces due to its hydrogen bonding.
Conclusion
As such, the correct increasing order of surface tension is:
- Hexane (III) < ethanol="" (ii)="" />< water="" />="" this="" leads="" to="" the="" conclusion="" that="" the="" arrangement="" in="" increasing="" order="" of="" surface="" tension="" is:="">Hexane (III) < ethanol="" (ii)="" />< water="" />
Thus, the correct answer is option 'D': III II I.
Surface tension is a physical property that describes the cohesive forces acting at the surface of a liquid. It is influenced by the type of molecules present and their interactions.
Comparison of Liquids
1. Water (I)
- High surface tension due to strong hydrogen bonding.
- Molecules are tightly held together, creating a robust surface.
2. Ethanol (II)
- Lower surface tension than water.
- Hydrogen bonds are present, but ethanol has a relatively weaker intermolecular force due to its smaller size compared to water.
3. Hexane (III)
- Lowest surface tension among the three.
- Non-polar molecules result in weaker van der Waals forces, leading to a less cohesive surface.
Order of Surface Tension
- Based on the strength of intermolecular forces:
- Hexane (III) has the weakest intermolecular forces.
- Ethanol (II) has moderate forces.
- Water (I) exhibits the strongest intermolecular forces due to its hydrogen bonding.
Conclusion
As such, the correct increasing order of surface tension is:
- Hexane (III) < ethanol="" (ii)="" />< water="" />="" this="" leads="" to="" the="" conclusion="" that="" the="" arrangement="" in="" increasing="" order="" of="" surface="" tension="" is:="">Hexane (III) < ethanol="" (ii)="" />< water="" />
Thus, the correct answer is option 'D': III II I.
Statement I : Liquids tend to have maximum number of molecules at their surface.
Statement II : Small liquid drops have spherical shape.
- a)Statement I is correct but Statement II is incorrect
- b)Both Statement I and Statement II are correct and Statement II is not the correct explanation of Statement I
- c)Both Statement l and Statement II are correct and Statement Il is the correct explanation of Statement I
- d)Statement II is correct but Statement I is incorrect
Correct answer is option 'B'. Can you explain this answer?
Statement I : Liquids tend to have maximum number of molecules at their surface.
Statement II : Small liquid drops have spherical shape.
Statement II : Small liquid drops have spherical shape.
a)
Statement I is correct but Statement II is incorrect
b)
Both Statement I and Statement II are correct and Statement II is not the correct explanation of Statement I
c)
Both Statement l and Statement II are correct and Statement Il is the correct explanation of Statement I
d)
Statement II is correct but Statement I is incorrect

|
Sanaya Menon answered |
Statement I: Liquids tend to have maximum number of molecules at their surface.
Statement II: Small liquid drops have spherical shape.
Explanation:
Statement I: Liquids tend to have maximum number of molecules at their surface.
This statement is correct. Liquids have a tendency to minimize their surface area due to intermolecular forces. The molecules at the surface of a liquid experience different forces compared to the molecules in the bulk of the liquid. The molecules in the bulk are surrounded by other molecules from all sides and are attracted equally in all directions. However, the molecules at the surface have unbalanced forces, as they are only attracted by the molecules below and around them. This leads to a net inward force, causing the liquid to minimize its surface area and form a droplet or spread out as a thin film.
Statement II: Small liquid drops have spherical shape.
This statement is also correct. When a liquid drop is small, the cohesive forces between its molecules dominate over the adhesive forces between the liquid and its surroundings. Cohesion refers to the attraction between molecules of the same substance, while adhesion refers to the attraction between molecules of different substances. The cohesive forces tend to pull the liquid molecules towards each other, resulting in a spherical shape. This is because a sphere has the minimum surface area for a given volume, allowing the liquid to minimize its surface energy.
Explanation of the relationship between the statements:
Both Statement I and Statement II are correct, but Statement II is not the correct explanation of Statement I. While both statements individually describe the behavior of liquids, they are not directly related to each other. Statement I explains the tendency of liquids to have a maximum number of molecules at their surface due to intermolecular forces, while Statement II describes the spherical shape of small liquid drops due to cohesive forces.
Therefore, the correct answer is option B: Both Statement I and Statement II are correct, and Statement II is not the correct explanation of Statement I.
Statement II: Small liquid drops have spherical shape.
Explanation:
Statement I: Liquids tend to have maximum number of molecules at their surface.
This statement is correct. Liquids have a tendency to minimize their surface area due to intermolecular forces. The molecules at the surface of a liquid experience different forces compared to the molecules in the bulk of the liquid. The molecules in the bulk are surrounded by other molecules from all sides and are attracted equally in all directions. However, the molecules at the surface have unbalanced forces, as they are only attracted by the molecules below and around them. This leads to a net inward force, causing the liquid to minimize its surface area and form a droplet or spread out as a thin film.
Statement II: Small liquid drops have spherical shape.
This statement is also correct. When a liquid drop is small, the cohesive forces between its molecules dominate over the adhesive forces between the liquid and its surroundings. Cohesion refers to the attraction between molecules of the same substance, while adhesion refers to the attraction between molecules of different substances. The cohesive forces tend to pull the liquid molecules towards each other, resulting in a spherical shape. This is because a sphere has the minimum surface area for a given volume, allowing the liquid to minimize its surface energy.
Explanation of the relationship between the statements:
Both Statement I and Statement II are correct, but Statement II is not the correct explanation of Statement I. While both statements individually describe the behavior of liquids, they are not directly related to each other. Statement I explains the tendency of liquids to have a maximum number of molecules at their surface due to intermolecular forces, while Statement II describes the spherical shape of small liquid drops due to cohesive forces.
Therefore, the correct answer is option B: Both Statement I and Statement II are correct, and Statement II is not the correct explanation of Statement I.
Which of the following are insoluble in water?- a)AgCI
- b)AgBr
- c)AgF
- d)Agl
Correct answer is option 'A,B,D'. Can you explain this answer?
Which of the following are insoluble in water?
a)
AgCI
b)
AgBr
c)
AgF
d)
Agl

|
Ashwini Chakraborty answered |
Fluorides have abnormal solubilities than other halides.
Direction (Q. Nos. 11-13) This section contains a paragraph, wach describing theory, experiments, data etc. three Questions related to paragraph have been given.Each question have only one correct answer among the four given options (a),(b),(c),(d).Variation of viscosity (q) with temperature T is given by 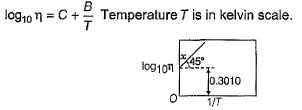 Q. What is viscosity at 100 K?
Q. What is viscosity at 100 K?- a)2.0500
- b)0.3117
- c)1.0000
- d)1.0200
Correct answer is option 'B'. Can you explain this answer?
Direction (Q. Nos. 11-13) This section contains a paragraph, wach describing theory, experiments, data etc. three Questions related to paragraph have been given.Each question have only one correct answer among the four given options (a),(b),(c),(d).
Variation of viscosity (q) with temperature T is given by
Q. What is viscosity at 100 K?
a)
2.0500
b)
0.3117
c)
1.0000
d)
1.0200
|
|
Puja Pillai answered |
From the graph, we can see that;
log10 ƞ = 0.3010 + 1/T
= 0.3010 + 1/100 (at 100K) -------------(i)
Given that variation of viscosity(q) = log10ƞ with T
Therefore, log10 ƞ = 0.311
As only for log10 ƞ = 0.311, we will have eqn i correct
log10 ƞ = 0.3010 + 1/T
= 0.3010 + 1/100 (at 100K) -------------(i)
Given that variation of viscosity(q) = log10ƞ with T
Therefore, log10 ƞ = 0.311
As only for log10 ƞ = 0.311, we will have eqn i correct
Consider the following statements.
1. The viscosity of a gas increases with rise in temperature.
2. The viscosity of the liquid falls very rapidly with rise in temperature.
Which of the statements(s) given above is/are correct?- a)Only 1
- b)Only 2
- c)Both 1 and 2
- d)Neither 1 nor 2
Correct answer is option 'C'. Can you explain this answer?
Consider the following statements.
1. The viscosity of a gas increases with rise in temperature.
2. The viscosity of the liquid falls very rapidly with rise in temperature.
Which of the statements(s) given above is/are correct?
1. The viscosity of a gas increases with rise in temperature.
2. The viscosity of the liquid falls very rapidly with rise in temperature.
Which of the statements(s) given above is/are correct?
a)
Only 1
b)
Only 2
c)
Both 1 and 2
d)
Neither 1 nor 2
|
|
Rajesh Gupta answered |
With increase in temperature, viscosity increases in gases while it decreases in liquids. So both the statements are correct.
One of the following properties increase with increase in temperature,- a)Viscosity
- b)Surface tension
- c)Vapour pressure
- d)Density
Correct answer is option 'C'. Can you explain this answer?
One of the following properties increase with increase in temperature,
a)
Viscosity
b)
Surface tension
c)
Vapour pressure
d)
Density
|
|
Naina Bansal answered |
Vapor pressure or equilibrium vapor pressure is defined as the pressure exerted by a vapor in thermodynamic equilibrium with its condensed phases at a given temperature in a closed system. The equilibrium vapor pressure is an indication of a liquid's evaporation rate.
Direction (Q. Nos. 7-10) This section contains 4 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THANT ONE is correct.
Q. Select the correct alternate(s).
- a)Gases can be compressed easily
- b)Lack of compressibility is a characteristic property of liquids and solids
- c)Oxygen can dissolve In water because a force of attraction exists between water's perm anent dipoie moment and the induced dipole in O2
- d)London dispersion forces are the only intermolecular forces that allow non-poiar molecules to interact
Correct answer is option 'A,B,C,D'. Can you explain this answer?
Direction (Q. Nos. 7-10) This section contains 4 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THANT ONE is correct.
Q. Select the correct alternate(s).
a)
Gases can be compressed easily
b)
Lack of compressibility is a characteristic property of liquids and solids
c)
Oxygen can dissolve In water because a force of attraction exists between water's perm anent dipoie moment and the induced dipole in O2
d)
London dispersion forces are the only intermolecular forces that allow non-poiar molecules to interact
|
|
Jithin Saini answered |
All of the above are the properties of gases.
A chemist decides to find the vapour pressure of water by the gas saturation method . 100 L of N2 gas is passed through 65.44 g of water. After passage of the gas, 63.13 g water remained. The temperature of water is 298 K.Thus, vapour pressure of water is- a)23.86 mm
- b)652.13 mm
- c)676 mm
- d)736.14 mm
Correct answer is option 'A'. Can you explain this answer?
A chemist decides to find the vapour pressure of water by the gas saturation method . 100 L of N2 gas is passed through 65.44 g of water. After passage of the gas, 63.13 g water remained. The temperature of water is 298 K.
Thus, vapour pressure of water is
a)
23.86 mm
b)
652.13 mm
c)
676 mm
d)
736.14 mm
|
|
Tanishq Tiwari answered |
Given:
- Initial weight of water = 65.44 g
- Final weight of water = 63.13 g
- Volume of N2 gas passed = 100 L
- Temperature of water = 298 K
To find:
Vapour pressure of water
Solution:
Step 1: Calculate the moles of N2 gas passed
The volume of N2 gas passed is given as 100 L. Since the gas is at room temperature and pressure, we can use the ideal gas law to calculate the moles of N2 gas.
PV = nRT
Where:
P = pressure (unknown)
V = volume of gas (100 L)
n = moles of gas (unknown)
R = ideal gas constant (0.0821 L atm/mol K)
T = temperature (298 K)
Solving for n, we get:
n = PV / RT
Substituting the given values, we get:
n = (P * 100) / (0.0821 * 298)
Step 2: Calculate the moles of water vaporized
The change in weight of water can be used to calculate the moles of water vaporized.
Initial weight of water = 65.44 g
Final weight of water = 63.13 g
Change in weight = Initial weight - Final weight
= 65.44 g - 63.13 g
= 2.31 g
Since the molar mass of water is 18 g/mol, we can calculate the moles of water vaporized using the formula:
moles = weight / molar mass
Substituting the given values, we get:
moles = 2.31 g / 18 g/mol
Step 3: Calculate the vapour pressure of water
The vapour pressure of water can be calculated using the formula:
vapour pressure = moles of water vaporized / moles of N2 gas passed
Substituting the calculated values, we get:
vapour pressure = (2.31 g / 18 g/mol) / n
Now, substitute the value of 'n' calculated in Step 1 to get the final answer.
Final Answer:
The vapour pressure of water is approximately 23.86 mm. (Option A)
- Initial weight of water = 65.44 g
- Final weight of water = 63.13 g
- Volume of N2 gas passed = 100 L
- Temperature of water = 298 K
To find:
Vapour pressure of water
Solution:
Step 1: Calculate the moles of N2 gas passed
The volume of N2 gas passed is given as 100 L. Since the gas is at room temperature and pressure, we can use the ideal gas law to calculate the moles of N2 gas.
PV = nRT
Where:
P = pressure (unknown)
V = volume of gas (100 L)
n = moles of gas (unknown)
R = ideal gas constant (0.0821 L atm/mol K)
T = temperature (298 K)
Solving for n, we get:
n = PV / RT
Substituting the given values, we get:
n = (P * 100) / (0.0821 * 298)
Step 2: Calculate the moles of water vaporized
The change in weight of water can be used to calculate the moles of water vaporized.
Initial weight of water = 65.44 g
Final weight of water = 63.13 g
Change in weight = Initial weight - Final weight
= 65.44 g - 63.13 g
= 2.31 g
Since the molar mass of water is 18 g/mol, we can calculate the moles of water vaporized using the formula:
moles = weight / molar mass
Substituting the given values, we get:
moles = 2.31 g / 18 g/mol
Step 3: Calculate the vapour pressure of water
The vapour pressure of water can be calculated using the formula:
vapour pressure = moles of water vaporized / moles of N2 gas passed
Substituting the calculated values, we get:
vapour pressure = (2.31 g / 18 g/mol) / n
Now, substitute the value of 'n' calculated in Step 1 to get the final answer.
Final Answer:
The vapour pressure of water is approximately 23.86 mm. (Option A)
The molecular velocity of any gas is - [aieee-2011]- a)Inversely proportional to absolute temperature
- b)Directly proportional to square of temperature
- c)Directly proportional to square root of temperature
- d)Inversely proportional to the square root of temperature
Correct answer is option 'C'. Can you explain this answer?
The molecular velocity of any gas is - [aieee-2011]
a)
Inversely proportional to absolute temperature
b)
Directly proportional to square of temperature
c)
Directly proportional to square root of temperature
d)
Inversely proportional to the square root of temperature
|
|
Rohit Shah answered |
The average kinetic energy of a gas particle is directly proportional to the temperature. An increase in temperature increases the speed in which the gas molecules move. All gases at a given temperature have the same average kinetic energy. Lighter gas molecules move faster than heavier molecules.
For gaseous state, if most probable speed is denoted by C*, average speed by  and mean square speed by C, then for a large number of molecules the ratios of these speeds are : [Jee(Main) 2013, 3/120]
and mean square speed by C, then for a large number of molecules the ratios of these speeds are : [Jee(Main) 2013, 3/120]- a)C* :
 : C = 1.225 : 1.128 : 1
: C = 1.225 : 1.128 : 1 - b)C* :
 : C = 1.228 : 1.125 : 1
: C = 1.228 : 1.125 : 1 - c)C* :
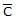 : C = 1 : 1.128 : 1.225
: C = 1 : 1.128 : 1.225 - d)C* :
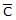 : C = 1 : 1.225 : 1.128
: C = 1 : 1.225 : 1.128
Correct answer is option 'C'. Can you explain this answer?
For gaseous state, if most probable speed is denoted by C*, average speed by  and mean square speed by C, then for a large number of molecules the ratios of these speeds are :
and mean square speed by C, then for a large number of molecules the ratios of these speeds are :
[Jee(Main) 2013, 3/120]
a)
C* : 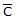 : C = 1.225 : 1.128 : 1
: C = 1.225 : 1.128 : 1
b)
C* :  : C = 1.228 : 1.125 : 1
: C = 1.228 : 1.125 : 1
c)
C* : 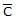 : C = 1 : 1.128 : 1.225
: C = 1 : 1.128 : 1.225
d)
C* : 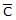 : C = 1 : 1.225 : 1.128
: C = 1 : 1.225 : 1.128

|
Knowledge Hub answered |
Direction (Q. Nos. 10-12) This section contains 3 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THANT ONE is correct.Q. Select correct statement(s).- a)The water drop in vacuum is perfectly spherical
- b)The shape of water drop is distorted due to action of gravity
- c)Soaps and detergents drastically decrease the surface tension of water
- d)Due to increase in temperature, surface tension also increases and becomes maximum at critical temperature.
Correct answer is option 'A,B,C'. Can you explain this answer?
Direction (Q. Nos. 10-12) This section contains 3 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THANT ONE is correct.
Q. Select correct statement(s).
a)
The water drop in vacuum is perfectly spherical
b)
The shape of water drop is distorted due to action of gravity
c)
Soaps and detergents drastically decrease the surface tension of water
d)
Due to increase in temperature, surface tension also increases and becomes maximum at critical temperature.
|
|
Saumya Dey answered |
The correct statements are (a), (b), and (c).
Let's discuss each statement in detail:
a) The water drop in vacuum is perfectly spherical:
When a water drop is in a vacuum, there is no air resistance or external forces acting on it. As a result, the water drop takes up a spherical shape, which is the shape that minimizes surface area for a given volume. This happens because the cohesive forces between water molecules pull them inward, creating a spherical shape. Any external forces, such as air resistance or gravity, can distort the shape of the water drop.
b) The shape of the water drop is distorted due to the action of gravity:
In the presence of gravity, the water drop is subject to the force of gravity pulling it downward. As a result, the bottom of the water drop gets flattened, and the shape becomes distorted. The force of gravity is stronger on the lower part of the water drop, causing it to spread out and create a flattened shape.
c) Soaps and detergents drastically decrease the surface tension of water:
Surface tension is the property of a liquid that allows it to resist an external force and minimize its surface area. Water has a high surface tension due to the cohesive forces between water molecules. However, when soaps and detergents are added to water, they disrupt these cohesive forces and decrease the surface tension. This is why soap bubbles can form and why water spreads more easily on surfaces treated with soap or detergent.
d) Due to an increase in temperature, surface tension also increases and becomes maximum at the critical temperature:
This statement is incorrect. In general, as the temperature of a liquid increases, the surface tension decreases. This is because an increase in temperature increases the kinetic energy of the molecules, causing them to move more rapidly and overcome the cohesive forces that create surface tension. The critical temperature is the temperature above which a substance cannot exist in the liquid state, regardless of pressure. It is not related to the surface tension behavior with temperature.
Let's discuss each statement in detail:
a) The water drop in vacuum is perfectly spherical:
When a water drop is in a vacuum, there is no air resistance or external forces acting on it. As a result, the water drop takes up a spherical shape, which is the shape that minimizes surface area for a given volume. This happens because the cohesive forces between water molecules pull them inward, creating a spherical shape. Any external forces, such as air resistance or gravity, can distort the shape of the water drop.
b) The shape of the water drop is distorted due to the action of gravity:
In the presence of gravity, the water drop is subject to the force of gravity pulling it downward. As a result, the bottom of the water drop gets flattened, and the shape becomes distorted. The force of gravity is stronger on the lower part of the water drop, causing it to spread out and create a flattened shape.
c) Soaps and detergents drastically decrease the surface tension of water:
Surface tension is the property of a liquid that allows it to resist an external force and minimize its surface area. Water has a high surface tension due to the cohesive forces between water molecules. However, when soaps and detergents are added to water, they disrupt these cohesive forces and decrease the surface tension. This is why soap bubbles can form and why water spreads more easily on surfaces treated with soap or detergent.
d) Due to an increase in temperature, surface tension also increases and becomes maximum at the critical temperature:
This statement is incorrect. In general, as the temperature of a liquid increases, the surface tension decreases. This is because an increase in temperature increases the kinetic energy of the molecules, causing them to move more rapidly and overcome the cohesive forces that create surface tension. The critical temperature is the temperature above which a substance cannot exist in the liquid state, regardless of pressure. It is not related to the surface tension behavior with temperature.
Direction (Q. Nos. 16-20) This section contains 5 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct.Q. Identify the products formed at the electrodes upon electrolysis of molten ICI.- a)I2 at cathode
- b)Cl2 at anode
- c)I2 and Cl2 at anode
- d) Cl2 at cathode
Correct answer is option 'A,C'. Can you explain this answer?
Direction (Q. Nos. 16-20) This section contains 5 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct.
Q.
Identify the products formed at the electrodes upon electrolysis of molten ICI.
a)
I2 at cathode
b)
Cl2 at anode
c)
I2 and Cl2 at anode
d)
Cl2 at cathode

|
Anuj Iyer answered |
Direction (Q. Nos. 10-11) This section contains 4 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THANT ONE is correct.Q. HF molecule also exist in liquid state. Types of intermolecular forces between them a- a)intermolecular H-bonding
- b) intramolecular H-bonding
- c)dipole-dipole interaction
- d)London forces of attraction
Correct answer is option 'A,C'. Can you explain this answer?
Direction (Q. Nos. 10-11) This section contains 4 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THANT ONE is correct.
Q. HF molecule also exist in liquid state. Types of intermolecular forces between them a
a)
intermolecular H-bonding
b)
intramolecular H-bonding
c)
dipole-dipole interaction
d)
London forces of attraction
|
|
Kirti Choudhary answered |
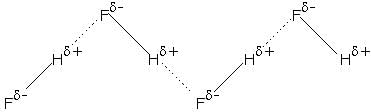
Here, both dipole-dipole interaction and intermolecular H-bonding takes place which makes HF a liquid.
Statement I : A certain amount of energy called activation energy (Ea) is required to move into a hole.Statement II : On increasing temperature, Ea is available and liquid can flow more easily thus, viscosity decreases.- a)Both Statement l and Statement II are correct and Statement Il is the correct explanation of Statement I
- b)Both Statement I and Statement II are correct and Statement II is not the correct explanation of Statement I
- c)Statement I is correct but Statement II is incorrect
- d)Statement II is correct but Statement I is incorrect
Correct answer is option 'A'. Can you explain this answer?
Statement I : A certain amount of energy called activation energy (Ea) is required to move into a hole.
Statement II : On increasing temperature, Ea is available and liquid can flow more easily thus, viscosity decreases.
a)
Both Statement l and Statement II are correct and Statement Il is the correct explanation of Statement I
b)
Both Statement I and Statement II are correct and Statement II is not the correct explanation of Statement I
c)
Statement I is correct but Statement II is incorrect
d)
Statement II is correct but Statement I is incorrect

|
Ashish Mishra answered |
A is correct.
Stronger intermolecular forces result in higher boiling point. Strength of London forces increases with number of electrons in the molecule. Boiling point of  Q. HF has highest boiling point because of
Q. HF has highest boiling point because of- a)London interaction
- b)hydrogen bonding
- c)dipole-dipole interaction
- d)induced dipole
Correct answer is option 'B'. Can you explain this answer?
Stronger intermolecular forces result in higher boiling point. Strength of London forces increases with number of electrons in the molecule. Boiling point of
Q. HF has highest boiling point because of
a)
London interaction
b)
hydrogen bonding
c)
dipole-dipole interaction
d)
induced dipole

|
Vanshika Rastogi answered |
It is because of hydrogen bonding . Since F is the most electronegative element , its hydrogen bonding tendency is strongest which makes boiling point of HF increase.
Heat of vaporisation of H2O is 40.6 kJ mol-1. At a 4000 m attitude , the atmospheric pressure is about 0.605 atm. What boiling point would you expect for water under these conditions?- a)100oC
- b)90oC
- c)86.2oC
- d)56.2oC
Correct answer is option 'C'. Can you explain this answer?
Heat of vaporisation of H2O is 40.6 kJ mol-1. At a 4000 m attitude , the atmospheric pressure is about 0.605 atm. What boiling point would you expect for water under these conditions?
a)
100oC
b)
90oC
c)
86.2oC
d)
56.2oC
|
|
Siddharth Mehra answered |
Boiling Point of Water at 4000 m Altitude
Given:
- Heat of vaporisation of H2O is 40.6 kJ mol-1
- Atmospheric pressure at 4000 m altitude is about 0.605 atm
Concept:
- Boiling point is the temperature at which the vapor pressure of a liquid is equal to the atmospheric pressure.
- At higher altitudes, the atmospheric pressure decreases, which affects the boiling point of liquids.
Calculation:
1. We can use the Clausius-Clapeyron equation to relate the boiling point and the vapor pressure of a liquid.
- ln(P1/P2) = ΔHvap/R * (1/T2 - 1/T1)
- P1 and P2 are the vapor pressures at temperatures T1 and T2
- ΔHvap is the heat of vaporisation
- R is the gas constant
2. Let's assume the boiling point at sea level (1 atm) is 100°C (373 K). We need to find the boiling point at 4000 m altitude (0.605 atm).
3. Rearranging the equation, we have:
- ln(P2/1 atm) = ΔHvap/R * (1/T2 - 1/373 K)
- ln(0.605) = 40.6 kJ mol-1 / (8.314 J mol-1 K-1) * (1/T2 - 1/373 K)
4. Simplifying the equation:
- ln(0.605) = 4.899 * (1/T2 - 1/373)
5. Now, let's solve for T2 (boiling point at 4000 m altitude):
- ln(0.605) = 4.899 * (1/T2 - 1/373)
- ln(0.605) = 4.899/T2 - 4.899/373
- 4.899/T2 = ln(0.605) + 4.899/373
- 1/T2 = (ln(0.605) + 4.899/373) / 4.899
- T2 = 1 / [(ln(0.605) + 4.899/373) / 4.899]
6. Using a calculator, we find T2 ≈ 86.2°C.
Conclusion:
Therefore, the boiling point of water at 4000 m altitude (0.605 atm) is approximately 86.2°C. Hence, the correct answer is option C) 86.2°C.
Given:
- Heat of vaporisation of H2O is 40.6 kJ mol-1
- Atmospheric pressure at 4000 m altitude is about 0.605 atm
Concept:
- Boiling point is the temperature at which the vapor pressure of a liquid is equal to the atmospheric pressure.
- At higher altitudes, the atmospheric pressure decreases, which affects the boiling point of liquids.
Calculation:
1. We can use the Clausius-Clapeyron equation to relate the boiling point and the vapor pressure of a liquid.
- ln(P1/P2) = ΔHvap/R * (1/T2 - 1/T1)
- P1 and P2 are the vapor pressures at temperatures T1 and T2
- ΔHvap is the heat of vaporisation
- R is the gas constant
2. Let's assume the boiling point at sea level (1 atm) is 100°C (373 K). We need to find the boiling point at 4000 m altitude (0.605 atm).
3. Rearranging the equation, we have:
- ln(P2/1 atm) = ΔHvap/R * (1/T2 - 1/373 K)
- ln(0.605) = 40.6 kJ mol-1 / (8.314 J mol-1 K-1) * (1/T2 - 1/373 K)
4. Simplifying the equation:
- ln(0.605) = 4.899 * (1/T2 - 1/373)
5. Now, let's solve for T2 (boiling point at 4000 m altitude):
- ln(0.605) = 4.899 * (1/T2 - 1/373)
- ln(0.605) = 4.899/T2 - 4.899/373
- 4.899/T2 = ln(0.605) + 4.899/373
- 1/T2 = (ln(0.605) + 4.899/373) / 4.899
- T2 = 1 / [(ln(0.605) + 4.899/373) / 4.899]
6. Using a calculator, we find T2 ≈ 86.2°C.
Conclusion:
Therefore, the boiling point of water at 4000 m altitude (0.605 atm) is approximately 86.2°C. Hence, the correct answer is option C) 86.2°C.
Statement I : As temperature is increased, less work is required to extend the surface of a liquid therefore surface tension decreases.Statement II : As the temperature and hence the intensity of molecular motion increases, intermolecular forces become less effective.- a)Both Statement l and Statement II are correct and Statement Il is the correct explanation of Statement I
- b)Both Statement I and Statement II are correct and Statement II is not the correct explanation of Statement I
- c)Statement I is correct but Statement II is incorrect
- d)Statement II is correct but Statement I is incorrect
Correct answer is option 'A'. Can you explain this answer?
Statement I : As temperature is increased, less work is required to extend the surface of a liquid therefore surface tension decreases.
Statement II : As the temperature and hence the intensity of molecular motion increases, intermolecular forces become less effective.
a)
Both Statement l and Statement II are correct and Statement Il is the correct explanation of Statement I
b)
Both Statement I and Statement II are correct and Statement II is not the correct explanation of Statement I
c)
Statement I is correct but Statement II is incorrect
d)
Statement II is correct but Statement I is incorrect

|
Ashish Mishra answered |
A is correct
How does the surface tension of a liquid vary with increase in temperature?- a)Remains same
- b)Decreases
- c)Increases
- d)No regular pattern is followed
Correct answer is option 'B'. Can you explain this answer?
How does the surface tension of a liquid vary with increase in temperature?
a)
Remains same
b)
Decreases
c)
Increases
d)
No regular pattern is followed
|
|
Naina Bansal answered |
In general, surface tension decreases when temperature increases because cohesive forces decrease with an increase of molecular thermal activity. The influence of the surrounding environment is due to the adhesive action liquid molecules have at the interface.
Chapter doubts & questions for Liquid State - 6 Months Preparation for JEE 2025 is part of JEE exam preparation. The chapters have been prepared according to the JEE exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for JEE 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Liquid State - 6 Months Preparation for JEE in English & Hindi are available as part of JEE exam.
Download more important topics, notes, lectures and mock test series for JEE Exam by signing up for free.

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up
within 7 days!
within 7 days!
Takes less than 10 seconds to signup