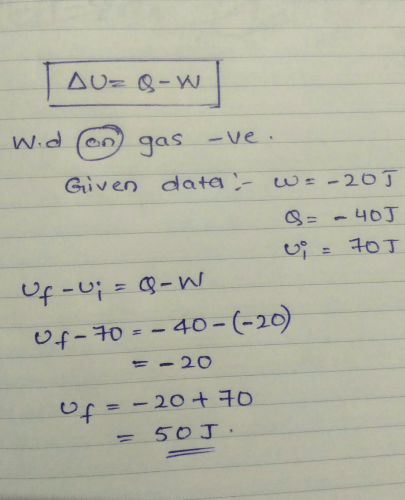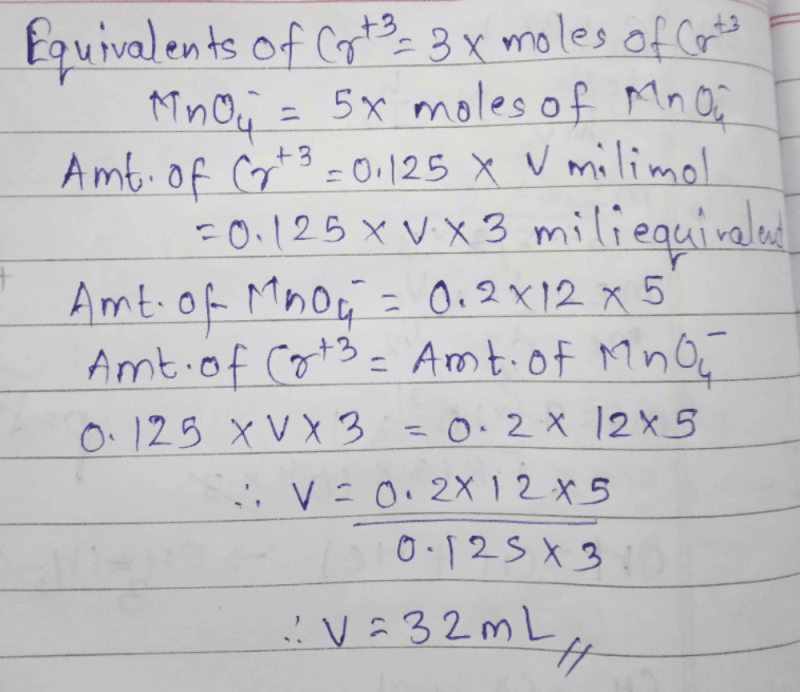All questions of 2013 for NEET Exam
The value of g at a particular point is 9.8 ms-2 Suppose the earth suddenly shrinks uniformly to half it resent size without losing any mass . The value of g at the same point (distance of the point from the centre of earth does not change) Will now be- a)9.8 ms-2
- b)4.9 ms-2
- c)19.6 ms-2
- d)39.2 ms-2
Correct answer is option 'A'. Can you explain this answer?

|
Khushi Mittal answered |
Can you explain the answer of this question below:Directions: These questions consist of two statements each printed as assertion and reason. Whole answering these questions you are required to choose any one of of following responses.
Q.
Assertion Tropical rain forest are nth in flora and fauna along with microbes on this biosphere.
Reason The low latitude humid tropics harbour the rainforest ecosystem.
- A:
Both Assertion and Reason are true and Reason is the correct explanation of Assertion
- B:
Both Assertion and Reason are true but Reason is not the correct explanation of Assertion
- C:
Assertion is true but Reason is false.
- D:
Both Assertion and Reason are false
The answer is a.
Directions: These questions consist of two statements each printed as assertion and reason. Whole answering these questions you are required to choose any one of of following responses.
Q.
Assertion Tropical rain forest are nth in flora and fauna along with microbes on this biosphere.
Reason The low latitude humid tropics harbour the rainforest ecosystem.
Both Assertion and Reason are true and Reason is the correct explanation of Assertion
Both Assertion and Reason are true but Reason is not the correct explanation of Assertion
Assertion is true but Reason is false.
Both Assertion and Reason are false

|
Researcher Guru. answered |
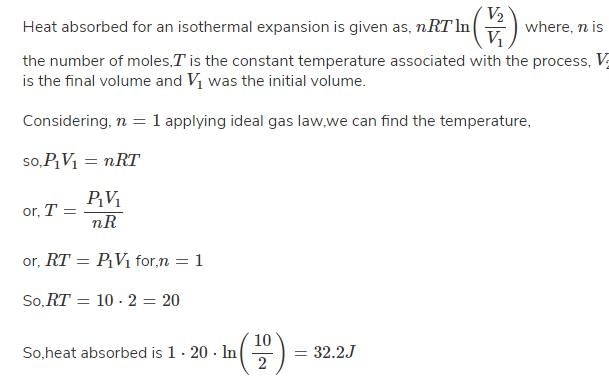
For the reaction, 1 g mole of CaCO3 is enclosed in 5 L container CaCO3(s) → CaO(s) CO2(g)Kp = 1.16 at 1073 K then per cent dissociation of CaCO3 is- a)zero
- b)6.58%
- c)65%
- d)100%
Correct answer is option 'B'. Can you explain this answer?
|
|
Sarita Koturwar answered |
%Dissociation =√Kp/P ×100
Can you explain the answer of this question below:Sound waves do not.show the phenomenon of
- A:
interference
- B:
diffraction
- C:
refraction
- D:
polarisation
- E:
refraction(d) polarisation
The answer is d.
Sound waves do not.show the phenomenon of
interference
diffraction
refraction
polarisation
refraction(d) polarisation

|
Jemimà Fràñcis answered |
Using the data given below find out the strongest reducing agentE°Cr2O4 /Cr3+ = 1.33 V, E°Cl2 / Cl- = 1.36 V,
E°MnO4- /Mn2+ = 1.51 V, E°Cr3+ /Cr = -0.74 V - a)Cl-
- b)Mn2+
- c)Cr
- d)Cr3+
Correct answer is option 'C'. Can you explain this answer?
E°MnO4- /Mn2+ = 1.51 V, E°Cr3+ /Cr = -0.74 V

|
Mohd Haris Ansari answered |
A slab consist of two portions of different materials of same thickness and having the conductivities K1 and K2. The equivalent thermal conductivity of the slab is

- a)a
- b)b
- c)c
- d)d
Correct answer is option 'C'. Can you explain this answer?


|
Moumita Khanna answered |
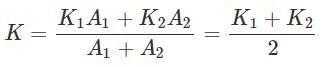 (As A1=A2).
(As A1=A2).In the preparation of HNO3 we get NO gas by catalytic oxidation of ammonia. The moles of NO produced by the oxidation of two moles of NH3 will be- a)2
- b)3
- c)4
- d)6
Correct answer is option 'A'. Can you explain this answer?
|
|
Rohit Das answered |
**Catalytic Oxidation of Ammonia:**
In the preparation of HNO3, ammonia (NH3) is oxidized to nitrogen monoxide (NO) gas by catalytic oxidation. The reaction can be represented as follows:
4NH3 + 5O2 → 4NO + 6H2O
**Mole Ratio:**
From the balanced chemical equation, we can determine the mole ratio between NH3 and NO.
- According to the balanced equation, 4 moles of NH3 react to produce 4 moles of NO.
- Therefore, the mole ratio of NH3 to NO is 4:4, which simplifies to 1:1.
**Moles of NO produced:**
Given that we are oxidizing 2 moles of NH3, we can use the mole ratio to determine the moles of NO produced.
- Since the mole ratio of NH3 to NO is 1:1, the moles of NO produced will also be 2.
Therefore, the correct answer is option A: 2 moles of NO are produced by the oxidation of two moles of NH3.
Which one plant movement is unidirectional?- a)Phototaxis
- b)Chemotaxis
- c)Both (a) and (b)
- d)Thigmotrophism
Correct answer is option 'C'. Can you explain this answer?
|
|
Amrutha Yadav answered |
Phototaxis and chemotaxis are both plant movements that involve the directional response to environmental stimuli.
Phototaxis:
- Phototaxis is the movement of an organism in response to light stimulus.
- In plants, phototaxis involves the growth of plant parts towards light sources to maximize photosynthesis.
- This movement is unidirectional as plants grow towards the light source.
Chemotaxis:
- Chemotaxis is the movement of an organism in response to chemical stimuli.
- In plants, chemotaxis can involve the growth of roots towards nutrients in the soil.
- This movement is also unidirectional as plants grow towards the source of the chemical stimulus.
Both (a) and (b):
- Both phototaxis and chemotaxis are examples of unidirectional plant movements where the plant parts grow in a specific direction in response to the stimuli.
Thigmotrophism:
- Thigmotrophism is another type of plant movement in response to touch or contact stimuli.
- Unlike phototaxis and chemotaxis, thigmotrophism can involve bidirectional responses where the plant parts can grow towards or away from the touch stimulus.
In conclusion, phototaxis and chemotaxis are examples of unidirectional plant movements where the growth of plant parts is directed towards the source of the stimulus.
Can you explain the answer of this question below:Flame cells and Malpighian tubules are the analogous organ in
- A:
insects and arthropods respectively
- B:
arthropods and echinodermates respectively
- C:
helminths and arthropods with other insect respectively
- D:
arthropods and other insect with helminths respectively
The answer is c.
Flame cells and Malpighian tubules are the analogous organ in
insects and arthropods respectively
arthropods and echinodermates respectively
helminths and arthropods with other insect respectively
arthropods and other insect with helminths respectively

|
Supriya Senapati answered |
These questions consist of two statements each printed as assertion and reason. Whole answering these questions you are required to choose any one of of following responses. Assertion : Plasmids are single stranded extrachromosomal DNA.
Reason : Plasmid are usually present in eukaryotic cells.- a)Both Assertion and Reason are true and Reason is the correct explanation of Assertion
- b)Both Assertion and Reason are true but Reason is not the correct explanation of Assertion
- c)Assertion is true but Reason is false.
- d)Both Assertion and Reason are false
Correct answer is option 'D'. Can you explain this answer?
Reason : Plasmid are usually present in eukaryotic cells.
|
|
Jyoti Aiims Aspirant answered |
Velocity of sound waves in air is 330 m/s. For a particular sound in air a path difference of 40 cm is equivalent to phase difference of 1.6π. The frequency of the wave is- a)165 Hz
- b)150 Hz
- c)660 Hz
- d)330 Hz
Correct answer is option 'C'. Can you explain this answer?

|
Surya answered |
Which one of the following bonds produces a solid that reflects light in the visible region and whose electrical conductivity decreases with temperature and hs high melting point?- a)metallic bonding
- b)van der Waal's bonding
- c)Ionic bonding
- d)covalent bonding
Correct answer is option 'A'. Can you explain this answer?

|
Shruti Bora answered |
Who had proposed theory of cohesion and adhesion forces?- a)Dixon and Jolly (1894)
- b)Dixon and Benson (1885)
- c)Dixon and Jolly (1950)
- d)Sir Jagdish Chandra Bose (1850)
Correct answer is option 'A'. Can you explain this answer?

|
Mamali . answered |
An alternatmg voltage V = V0 sin ωτ is applied across a circuit. As result I = I0sin(ωτ)- π/2) flows ill it. The power consumed per cycle is- a)zero
- b) 0.5 V0I0
- c) 0.707 V0I0
- d) 1.414 V0I0
Correct answer is option 'A'. Can you explain this answer?

|
Akhil George answered |
Two simple harmonic motions are represented by y1=4sin(πt– π/2) and y2=3cos(4πt). The resultant amplitude is- a)/
- b)1
- c)5
- d)2+√3
Correct answer is 'A'. Can you explain this answer?

|
Kanak Agrawal answered |
Different varieties of Indian mangoes are most popular in Western and some other European countries. The varieties with different flavour, colour, sugar and fleshy content is due to- a)genetic diversity
- b)species diversity
- c)induced mutation
- d)hybridisation
Correct answer is option 'A'. Can you explain this answer?

|
Ved Patidar answered |
In a child of 15 years age, plasma calcium level is diagnosed below optimum level. Which organ is malfunctioning?- a)Thyroid gland
- b)Liver
- c)Parathyroid
- d)Posterior lobe of pituitary
Correct answer is option 'C'. Can you explain this answer?

|
Nupur Juyal answered |
These questions consist of two statements each printed as assertion and reason. Whole answering these questions you are required to choose any one of of following responses.Assertion : In an elastic collision between two bodies. the energy of each body is conserved
Reason : The total energy of an isolated system is conserved.- a)If both the assertion and reason are true and reason explains the assertion
- b)If both the assertion and reason are true but reason does not explain the assertion
- c)If assertion is true but reason is false
- d)If assertion is false but reason is true
Correct answer is option 'D'. Can you explain this answer?
Reason : The total energy of an isolated system is conserved.

|
Abhimanyu K S answered |
The plants which can withstand with narrow and broad range of temperature tolerance respectively are- a)munotherrnai and stencthermal
- b)stenuthermal and monothermal
- c)stenothermal and eurythermal
- d)stenothermal and mesothermal
Correct answer is option 'C'. Can you explain this answer?

|
Kavya Biradar answered |
There are N cells in the circuit of figure. The emf and internal resistance of each cell is E and r respectively. The points A and B in the circuit divide the circuit into n and (N – n) cells. The current in the circuit is- a)E/r
- b)nE/r
- c)NE/Nr
- d)Zero
Correct answer is option 'A'. Can you explain this answer?
|
|
Ekta Gupta answered |
Flame cells and Malpighian tubules are the analogous organ in- a)insects and arthropods respectively
- b)arthropods and echinodermates respectively
- c)helminths and arthropods with other insect respectively
- d)arthropods and other insect with helminths respectively
Correct answer is option 'C'. Can you explain this answer?

|
Anmol answered |
Trochlear, trigeminal and glossopharyngeal nerve are respectively
a) IX, V and IV
b) IV, V and X
c) V, IV and IX
d) IV, V and IX
Correct answer is option 'D'. Can you explain this answer?
|
|
Shreya Singh answered |
These questions consist of two statements each printed as assertion and reason. Whole answering these questions you are required to choose any one of of following responses. Assertion : Medulla is considered as a respiratory centre in animals.
Reason : Rate of breathing is regulated by medulla because of the changes in O2content of blood.- a)Both Assertion and Reason are true and Reason is the correct explanation of Assertion
- b)Both Assertion and Reason are true but Reason is not the correct explanation of Assertion
- c)Assertion is true but Reason is false.
- d)Both Assertion and Reason are false
Correct answer is option 'A'. Can you explain this answer?
Reason : Rate of breathing is regulated by medulla because of the changes in O2content of blood.
|
|
Anchal Maurya answered |
These questions consist of two statements each printed as assertion and reason. Whole answering these questions you are required to choose any one of of following responses.Assertion : If a liquid in a vessel is stirred and left to itself. the motion disappear after few minutes.
Reason : The moving liquid exerts equal and opposite force.- a)If both the assertion and reason are true and reason explains the assertion
- b)If both the assertion and reason are true but reason does not explain the assertion
- c)If assertion is true but reason is false
- d)If assertion is false but reason is true
Correct answer is option 'C'. Can you explain this answer?
Reason : The moving liquid exerts equal and opposite force.
|
|
Anjali Chawla answered |
Chapter doubts & questions for 2013 - AIIMS Mock Tests & Previous Year Question Papers 2025 is part of NEET exam preparation. The chapters have been prepared according to the NEET exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for NEET 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
AIIMS Mock Tests & Previous Year Question Papers
3 videos|45 docs|66 tests
|