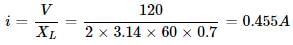All Exams >
JEE >
SRMJEEE Subject Wise & Full Length Mock Tests 2026 >
All Questions
All questions of Medical for JEE Exam
A low-loss transformer has 230 volts applied to the primary and gives 4.6 volts in the secondary. Secondary is connected to a load which draws 5 ampere of current. The current (in ampere) in the primary is- a)0.1
- b)1.0
- c)10
- d)250
Correct answer is option 'A'. Can you explain this answer?
A low-loss transformer has 230 volts applied to the primary and gives 4.6 volts in the secondary. Secondary is connected to a load which draws 5 ampere of current. The current (in ampere) in the primary is
a)
0.1
b)
1.0
c)
10
d)
250
|
|
Anand Sen answered |
Given:
Primary voltage (Vp) = 230 V
Secondary voltage (Vs) = 4.6 V
Secondary current (Is) = 5 A
To find: Primary current (Ip)
Step-by-Step Solution:
1. Calculate the turns ratio (N) of the transformer using the voltage ratio formula:
N = Vs / Vp = 4.6 / 230 = 0.02
2. Since the transformer is low-loss, we can assume that the power in the primary is equal to the power in the secondary:
Vp * Ip = Vs * Is
3. Substitute the values given and solve for Ip:
Ip = (Vs * Is) / Vp = (4.6 * 5) / 230 = 0.1 A
Therefore, the primary current is 0.1 A.
Answer: (a) 0.1
Primary voltage (Vp) = 230 V
Secondary voltage (Vs) = 4.6 V
Secondary current (Is) = 5 A
To find: Primary current (Ip)
Step-by-Step Solution:
1. Calculate the turns ratio (N) of the transformer using the voltage ratio formula:
N = Vs / Vp = 4.6 / 230 = 0.02
2. Since the transformer is low-loss, we can assume that the power in the primary is equal to the power in the secondary:
Vp * Ip = Vs * Is
3. Substitute the values given and solve for Ip:
Ip = (Vs * Is) / Vp = (4.6 * 5) / 230 = 0.1 A
Therefore, the primary current is 0.1 A.
Answer: (a) 0.1
At 27oC temperature, the kinetic energy of an ideal gas is E1. If the temperature is icnreased to 327oC, then kinetic energy would be- a)2E1
- b)1/2 E1
- c)√2 E1
- d)1/√2 E1
Correct answer is option 'A'. Can you explain this answer?
At 27oC temperature, the kinetic energy of an ideal gas is E1. If the temperature is icnreased to 327oC, then kinetic energy would be
a)
2E1
b)
1/2 E1
c)
√2 E1
d)
1/√2 E1
|
|
Hansa Sharma answered |
Average kinetic energy of gas molecules
E = 3/2 RT/N
R = ideal constant
N = Avogadro number
∴ E ∝ T
Hence, E1/E2 = T1/T2
E1/E2 = 27 + 273/327 + 273
E1/E2 = 300/600
∴ E2 = 2E1
E = 3/2 RT/N
R = ideal constant
N = Avogadro number
∴ E ∝ T
Hence, E1/E2 = T1/T2
E1/E2 = 27 + 273/327 + 273
E1/E2 = 300/600
∴ E2 = 2E1
Maximum number of active hydrogens are present in- a)Acetic acid
- b)Methane
- c)Glycerol
- d)Methanol
Correct answer is option 'C'. Can you explain this answer?
Maximum number of active hydrogens are present in
a)
Acetic acid
b)
Methane
c)
Glycerol
d)
Methanol
|
|
Bibek Unni answered |
Maximum Number of Active Hydrogens in Different Compounds
Acetic Acid (CH3COOH)
- Acetic acid has a chemical formula of CH3COOH.
- The carbon atom in the carboxyl group (COOH) is bonded to two oxygen atoms and has one hydrogen atom directly attached to it.
- The other carbon atom in the compound is bonded to three hydrogen atoms.
- Thus, acetic acid has a total of 4 active hydrogens: one from the carboxyl group and three from the methyl group.
Methane (CH4)
- Methane has a chemical formula of CH4.
- It consists of a single carbon atom bonded to four hydrogen atoms.
- All four hydrogen atoms in methane are directly attached to the carbon atom.
- Thus, methane has a total of 4 active hydrogens.
Glycerol (C3H8O3)
- Glycerol, also known as glycerin, has a chemical formula of C3H8O3.
- It consists of a chain of three carbon atoms, each bonded to two hydrogen atoms.
- One of the carbon atoms is also bonded to an oxygen atom, which forms a hydroxyl group (-OH).
- Glycerol has a total of 3 hydroxyl groups.
- Each hydroxyl group has one active hydrogen.
- Thus, glycerol has a total of 3 active hydrogens.
Methanol (CH3OH)
- Methanol has a chemical formula of CH3OH.
- It consists of a carbon atom bonded to three hydrogen atoms and one oxygen atom.
- The oxygen atom forms a hydroxyl group (-OH) with the carbon atom.
- The hydroxyl group has one active hydrogen.
- Additionally, the carbon atom is also bonded to three hydrogen atoms.
- Thus, methanol has a total of 4 active hydrogens.
Conclusion
- Among the given compounds, glycerol has the maximum number of active hydrogens with 3, followed by acetic acid and methanol with 4 each, and methane with 4 as well.
- Therefore, the correct answer is option 'C', glycerol.
Acetic Acid (CH3COOH)
- Acetic acid has a chemical formula of CH3COOH.
- The carbon atom in the carboxyl group (COOH) is bonded to two oxygen atoms and has one hydrogen atom directly attached to it.
- The other carbon atom in the compound is bonded to three hydrogen atoms.
- Thus, acetic acid has a total of 4 active hydrogens: one from the carboxyl group and three from the methyl group.
Methane (CH4)
- Methane has a chemical formula of CH4.
- It consists of a single carbon atom bonded to four hydrogen atoms.
- All four hydrogen atoms in methane are directly attached to the carbon atom.
- Thus, methane has a total of 4 active hydrogens.
Glycerol (C3H8O3)
- Glycerol, also known as glycerin, has a chemical formula of C3H8O3.
- It consists of a chain of three carbon atoms, each bonded to two hydrogen atoms.
- One of the carbon atoms is also bonded to an oxygen atom, which forms a hydroxyl group (-OH).
- Glycerol has a total of 3 hydroxyl groups.
- Each hydroxyl group has one active hydrogen.
- Thus, glycerol has a total of 3 active hydrogens.
Methanol (CH3OH)
- Methanol has a chemical formula of CH3OH.
- It consists of a carbon atom bonded to three hydrogen atoms and one oxygen atom.
- The oxygen atom forms a hydroxyl group (-OH) with the carbon atom.
- The hydroxyl group has one active hydrogen.
- Additionally, the carbon atom is also bonded to three hydrogen atoms.
- Thus, methanol has a total of 4 active hydrogens.
Conclusion
- Among the given compounds, glycerol has the maximum number of active hydrogens with 3, followed by acetic acid and methanol with 4 each, and methane with 4 as well.
- Therefore, the correct answer is option 'C', glycerol.
One mole of an ideal gas at 300 K is expanded isothermally from an initial volume of 1 litre to 10 litres. The ΔE for this process is (R = 2 cal.K-1 mol-1)- a)163.7 cal
- b)1381.1 cal
- c)9 lt atm
- d)zero
Correct answer is option 'D'. Can you explain this answer?
One mole of an ideal gas at 300 K is expanded isothermally from an initial volume of 1 litre to 10 litres. The ΔE for this process is (R = 2 cal.K-1 mol-1)
a)
163.7 cal
b)
1381.1 cal
c)
9 lt atm
d)
zero
|
|
Vicky Kumar answered |
Zero answer because in isothermal process, ∆T=0
and ∆E=NCv∆T ...
and ∆E=NCv∆T ...
The particles emitted by radioactive decay are deflected by magnetic field. The particles will be- a)Protons and α-particles
- b)Electrons, protons and α-particles
- c)Electrons, protons and neutrons
- d)Electrons and α-particles
Correct answer is option 'D'. Can you explain this answer?
The particles emitted by radioactive decay are deflected by magnetic field. The particles will be
a)
Protons and α-particles
b)
Electrons, protons and α-particles
c)
Electrons, protons and neutrons
d)
Electrons and α-particles
|
|
Mira Sharma answered |
Because they consist of charged particles, alpha radiation and beta radiation can also be deflected by magnetic fields. Just as with electric fields, gamma radiation is not deflected by magnetic fields.
pH of gastric juice is- a)2.0 - 3.0
- b)5.0 - 6.8
- c)7.0 - 9.0
- d)6.0 - 8.0
Correct answer is option 'A'. Can you explain this answer?
pH of gastric juice is
a)
2.0 - 3.0
b)
5.0 - 6.8
c)
7.0 - 9.0
d)
6.0 - 8.0
|
|
Khaja Moinuddin answered |
Gastric juice is highly acidic. Its pH is between 2.0 to 3.0
Which of the following types of hybridisation is involved in the formation of cycloalkanes?- a)sp3
- b)sp
- c)sp2
- d)sp3d2
Correct answer is option 'A'. Can you explain this answer?
Which of the following types of hybridisation is involved in the formation of cycloalkanes?
a)
sp3
b)
sp
c)
sp2
d)
sp3d2
|
|
Krishna Iyer answered |
The carbon atoms in cycloalkanes (also called naphthenes) are sp3 hybridized and are therefore a deviation from the ideal tetrahedral bond angles of 109o28'.
Which of the following is isoelectronic with carbon atom?- a)Na+
- b)Al3+
- c)O2 −
- d)N+
Correct answer is option 'D'. Can you explain this answer?
Which of the following is isoelectronic with carbon atom?
a)
Na+
b)
Al3+
c)
O2 −
d)
N+
|
|
Anaya Patel answered |
Isoelectronic species: a group of ions, atoms, or molecules that have the same number of electrons. Thus, carbon and N+ are isoelectronic with 6 electrons.
Delocalised electrons are present in- a)1,3-butadiene
- b)C6H6
- c)1,3,5,-hexatriene
- d)all of these
Correct answer is option 'D'. Can you explain this answer?
Delocalised electrons are present in
a)
1,3-butadiene
b)
C6H6
c)
1,3,5,-hexatriene
d)
all of these
|
|
Chirag Verma answered |
All the given compounds have conjugated system. A conjugated system is a system of connected p-orbitals with delocalized electrons in compounds with alternating single and multiple bonds.They allow a delocalization of pi electrons across all the adjacent aligned p-orbitals.The pi electrons do not belong to a single bond or atom, but rather to a group of atoms.
The specific function of light energy in the process of photosynthesis is to- a)Activate chlorophyll
- b)Split water
- c)Reduce carbon dioxide
- d)Synthesize glucose
Correct answer is option 'A'. Can you explain this answer?
The specific function of light energy in the process of photosynthesis is to
a)
Activate chlorophyll
b)
Split water
c)
Reduce carbon dioxide
d)
Synthesize glucose

|
Sagar Mukherjee answered |
Light serves a few roles in photosynthesis. It is energizes the chlorophylls, whic then transfer that energy to the reaction centers of the photosystems to initiate the transfer of an electron. Light is also used to signal the activation of the Calvin-Benson cycle (which is inhibited in the dark to conserve energy in the chloroplast) and to regulate many other developmental processes that affect photosynthesis. The synthesis of chlorophyll itself involves a light catalyzed reaction. In motile plants (such as swimming green algae), light is also used for navigation.
Which of the following is correctly matched?- a)C ― C bond length = 0.077 nm
- b)Ionic radius of Na+ = 0.136nm
- c)C ― Cl bond length = 0.176nm
- d)Ionic radius of F − = 0.095 nm
Correct answer is option 'C'. Can you explain this answer?
Which of the following is correctly matched?
a)
C ― C bond length = 0.077 nm
b)
Ionic radius of Na+ = 0.136nm
c)
C ― Cl bond length = 0.176nm
d)
Ionic radius of F − = 0.095 nm
|
|
Maitri Kumar answered |
C-Cl bond length is 0.176 nm; ionic radii of Na+ and F - are 0.095 and 0.136 nm respectively; C-C bond length is 0.154 nm.
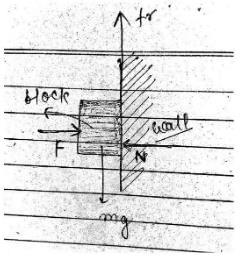
A block of 1 kg is stopped aganist a wall by aplying a force F perpendicular to the wall. If μ = 0.2 then minimum value of F will be- a)980 N
- b)49 N
- c)98 N
- d)490 N
Correct answer is option 'B'. Can you explain this answer?
A block of 1 kg is stopped aganist a wall by aplying a force F perpendicular to the wall. If μ = 0.2 then minimum value of F will be
a)
980 N
b)
49 N
c)
98 N
d)
490 N

|
Nikhil Sen answered |
Answer : 49 N
explanation : friction is the force resisting the relative motion of two solid surface. see figure, block has tendency to move downward with respect to wall.so, friction force must be applied upward as shown in figure.
a force F is applied perpendicularly on the block. Let normal reaction acts between the block and wall is N.
at equilibrium,
F = N...(1)
and fr = mg = 1kg x 9.8 m/s^2 = 9.8N
as we know, friction = μN, where μ is coefficient of friction.
so, 9.8 = 0.2 x N
or, N = 9.8/0.2 = 49
from equation (1),
F = 49N
hence, minimum value of F will be 49N
explanation : friction is the force resisting the relative motion of two solid surface. see figure, block has tendency to move downward with respect to wall.so, friction force must be applied upward as shown in figure.
a force F is applied perpendicularly on the block. Let normal reaction acts between the block and wall is N.
at equilibrium,
F = N...(1)
and fr = mg = 1kg x 9.8 m/s^2 = 9.8N
as we know, friction = μN, where μ is coefficient of friction.
so, 9.8 = 0.2 x N
or, N = 9.8/0.2 = 49
from equation (1),
F = 49N
hence, minimum value of F will be 49N
Given: P = 0.0030 m, Q = 2.40 m and R = 3,000 m. The significant figures in P,Q and R are respectively- a)2,2,1
- b)2,3,4
- c)4,2,1
- d)4,2,4
Correct answer is option 'B'. Can you explain this answer?
Given: P = 0.0030 m, Q = 2.40 m and R = 3,000 m. The significant figures in P,Q and R are respectively
a)
2,2,1
b)
2,3,4
c)
4,2,1
d)
4,2,4
|
|
Vignesh answered |
Signifiacant figures of P is 2 and Q is 3 R is 4
In a chemical equilibrium Kc = Kp when :- a)the number of molecules entering into the reaction is more than the number of molecules produced
- b)the total number of moles of the reactants equal that of the products
- c)the number of molecules entering into the reaction is the same as the number of molecules produced
- d)the number of molecules entering into the reaction is less than the number of molecules produced
Correct answer is option 'C'. Can you explain this answer?
In a chemical equilibrium Kc = Kp when :
a)
the number of molecules entering into the reaction is more than the number of molecules produced
b)
the total number of moles of the reactants equal that of the products
c)
the number of molecules entering into the reaction is the same as the number of molecules produced
d)
the number of molecules entering into the reaction is less than the number of molecules produced
|
|
Akshay Kapoor answered |
Explanation:
Chemical Equilibrium is a state in which the rate of forward reaction is equal to the rate of backward reaction. At equilibrium, the concentration of reactants and products remain constant.
Kc and Kp are equilibrium constants used to determine the concentration or partial pressure of reactants and products at equilibrium.
Kc - Equilibrium constant in terms of concentration
Kp - Equilibrium constant in terms of partial pressure
When does Kc = Kp?
Kc and Kp are related by the following equation:
Kp = Kc (RT)^(Δn)
where R is the gas constant, T is the temperature in Kelvin, and Δn is the change in the number of moles of gas molecules from reactant side to product side.
From the above equation, it is clear that Kc = Kp only when the number of gas molecules on the reactant side is equal to the number of gas molecules on the product side.
Therefore, the correct option is C) the number of molecules entering into the reaction is the same as the number of molecules produced.
Chemical Equilibrium is a state in which the rate of forward reaction is equal to the rate of backward reaction. At equilibrium, the concentration of reactants and products remain constant.
Kc and Kp are equilibrium constants used to determine the concentration or partial pressure of reactants and products at equilibrium.
Kc - Equilibrium constant in terms of concentration
Kp - Equilibrium constant in terms of partial pressure
When does Kc = Kp?
Kc and Kp are related by the following equation:
Kp = Kc (RT)^(Δn)
where R is the gas constant, T is the temperature in Kelvin, and Δn is the change in the number of moles of gas molecules from reactant side to product side.
From the above equation, it is clear that Kc = Kp only when the number of gas molecules on the reactant side is equal to the number of gas molecules on the product side.
Therefore, the correct option is C) the number of molecules entering into the reaction is the same as the number of molecules produced.
Forces of 1 and 2 units act along the lines x=0 and y=0. The equation of the line of action of the resultant is- a)y-2x=0
- b)2y-x=0
- c)y+x=0
- d)y-x=0
Correct answer is option 'B'. Can you explain this answer?
Forces of 1 and 2 units act along the lines x=0 and y=0. The equation of the line of action of the resultant is
a)
y-2x=0
b)
2y-x=0
c)
y+x=0
d)
y-x=0
|
|
Sahil Deshwal answered |
As eqn is reln in x and y cordinates thus we get this answer
The length of a given cylindrical wire is increased by 100%. Due to the consequent decrease in diameter the change in the resistance of the wire will be- a)300%
- b)200%
- c)100%
- d)50%
Correct answer is option 'A'. Can you explain this answer?
The length of a given cylindrical wire is increased by 100%. Due to the consequent decrease in diameter the change in the resistance of the wire will be
a)
300%
b)
200%
c)
100%
d)
50%
|
|
Ameya Das answered |
Since the volume will remain the same
And the wire is in the form of a cylinder
πr12l1 = πr22l2
so there fore
r12l1 = r22l2
so on l1 is the initial length, which is streched n times to the final length
l1 = l,,,,, l2 = nl
on substituing the values
And the wire is in the form of a cylinder
πr12l1 = πr22l2
so there fore
r12l1 = r22l2
so on l1 is the initial length, which is streched n times to the final length
l1 = l,,,,, l2 = nl
on substituing the values
R2 = n2R
Here in this question the value of n will be 2 as the length is increased by on substituing the value
R2 = 4R
Which is a 300% increase
Here in this question the value of n will be 2 as the length is increased by on substituing the value
R2 = 4R
Which is a 300% increase
If n capacitors each of capacitance C are connected in series with a battery of V volt, then electrostatic energy stored in all the capacitors will be- a)nCV2
- b)1 4 nCV2
- c)1 2n CV2
- d)None of these
Correct answer is option 'C'. Can you explain this answer?
If n capacitors each of capacitance C are connected in series with a battery of V volt, then electrostatic energy stored in all the capacitors will be
a)
nCV2
b)
1 4 nCV2
c)
1 2n CV2
d)
None of these
|
|
Devika Das answered |
The equivalent capacitance Cs of these series-connected capacitors is
1/C s = 1/C + 1/C + 1/C ..... n times = n/C
∴ Cs = C/ n
Energy stored in all capacitors = 1 2 CsV2 = 1/2 C/n V2 = 1/2n CV2
1/C s = 1/C + 1/C + 1/C ..... n times = n/C
∴ Cs = C/ n
Energy stored in all capacitors = 1 2 CsV2 = 1/2 C/n V2 = 1/2n CV2
On dissolving 1 mole of each of the following acids in 1 litre water, the acid does not give a solution of strength 1 N is- a)HCl
- b)Perchloric acid
- c)HNO₃
- d)Phosphoric acid
Correct answer is option 'B'. Can you explain this answer?
On dissolving 1 mole of each of the following acids in 1 litre water, the acid does not give a solution of strength 1 N is
a)
HCl
b)
Perchloric acid
c)
HNO₃
d)
Phosphoric acid
|
|
Raji Ram answered |
Correct answer is phosphoric acid as its n factor is 3
Dalton's law of partial pressure is not applicable to- a)O₂ + O₃
- b)CO + CO₂
- c)NH₃ + HCl
- d)I₂ + O₂
Correct answer is option 'C'. Can you explain this answer?
Dalton's law of partial pressure is not applicable to
a)
O₂ + O₃
b)
CO + CO₂
c)
NH₃ + HCl
d)
I₂ + O₂
|
|
Srinidhi V answered |
It's because Dalton's Law of Partial Pressure is applicable to only non-reacting species (gases) .
In the above mentioned options A, B, D gases do not react with each other.
But NH3 and HCl combine to form NH4Cl.
Hence Dalton's law isn't applicable for this!
In the above mentioned options A, B, D gases do not react with each other.
But NH3 and HCl combine to form NH4Cl.
Hence Dalton's law isn't applicable for this!
Reactance of a capacitor of capacitance C μ F for a.c. of frequency 400/π Hz is 25 Ω. The value of C is- a)25
- b)50
- c)400
- d)100
Correct answer is option 'B'. Can you explain this answer?
Reactance of a capacitor of capacitance C μ F for a.c. of frequency 400/π Hz is 25 Ω. The value of C is
a)
25
b)
50
c)
400
d)
100
|
|
Ayush Mishra answered |
Since, Xc=1÷wc
therefore, 25= 1/800C
w=2πf=2π×400/π=800
C=50microfarad
therefore, 25= 1/800C
w=2πf=2π×400/π=800
C=50microfarad
The plates of a parallel plate capacitor are charged upto 200 volts. A dielectric slab of thickness 4 mm is inserted between its plates. Then to maintain the same potential difference between the plates capacitor, the distance between the plates is increased by 3.2 mm. The dielectric constant of dielectric slab is- a)1
- b)4
- c)5
- d)6
Correct answer is option 'C'. Can you explain this answer?
The plates of a parallel plate capacitor are charged upto 200 volts. A dielectric slab of thickness 4 mm is inserted between its plates. Then to maintain the same potential difference between the plates capacitor, the distance between the plates is increased by 3.2 mm. The dielectric constant of dielectric slab is
a)
1
b)
4
c)
5
d)
6
|
|
Jayant Mishra answered |
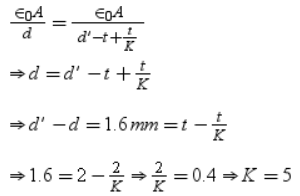
Which of the following is the correct order of ionic radii?- a)Na+ < Mg2+ < Al3+ < Si4+
- b)Al3+ < Si4+ < Na+ < Mg2+
- c)Si4+ < Al3+ > Mg2+ > Na+
- d)Na+ > Mg2+ > Al3+ > Si4+
Correct answer is option 'D'. Can you explain this answer?
Which of the following is the correct order of ionic radii?
a)
Na+ < Mg2+ < Al3+ < Si4+
b)
Al3+ < Si4+ < Na+ < Mg2+
c)
Si4+ < Al3+ > Mg2+ > Na+
d)
Na+ > Mg2+ > Al3+ > Si4+
|
|
Yash Modi answered |
All ions shown have the same number of electrons i.e. 18. But the number of protons are different. Thus more the number of protons, more is the attractive force experienced by the electrons and consequently smaller is the ionic radius of the ion. Thus the order becomes as shown in option D. (14 protons of Si(4+) attract 18 electrons more effectively than 11 protons of Na(+)).
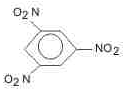
The major product (70%-80%) of reaction between m-dinitrobenzene with (NH4)2Sx is- a)
- b)
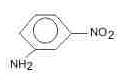
- c)
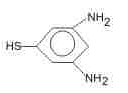
- d)
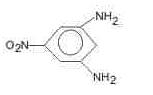
Correct answer is option 'B'. Can you explain this answer?
The major product (70%-80%) of reaction between m-dinitrobenzene with (NH4)2Sx is
a)
b)
c)
d)
|
|
Tejas Verma answered |
(NH4)2Sx reduces one - NO2 to - NH2 group
The amount of heat required to raise the temperature of a body temperature through 1oC is called, its- a)entropy
- b)molar heat
- c)specific heat
- d)thermal capacity
Correct answer is option 'C'. Can you explain this answer?
The amount of heat required to raise the temperature of a body temperature through 1oC is called, its
a)
entropy
b)
molar heat
c)
specific heat
d)
thermal capacity
|
|
Deepika Chavan answered |
Specific Heat
Specific heat is the amount of heat required to raise the temperature of a unit mass of a substance by 1 degree Celsius. It is denoted by the symbol 'C' and is expressed in J/kg°C or cal/g°C.
Explanation
- When heat is added to a substance, its temperature increases. The specific heat of a substance determines how much heat energy is required to raise its temperature.
- Different substances have different specific heat values due to their molecular structure and composition. For example, water has a high specific heat capacity compared to metals.
- The specific heat of a substance is an important property in various fields such as thermodynamics, chemistry, and engineering as it helps in calculating the amount of heat required for temperature changes.
- The specific heat capacity of water is particularly important in nature as it helps regulate the temperature of oceans and bodies of water, which in turn influences weather patterns and climate.
Importance
- Specific heat plays a crucial role in various applications, including designing heating and cooling systems, calculating energy requirements, and understanding the behavior of substances under different temperature conditions.
- It is a fundamental property used in scientific research, industrial processes, and everyday life to determine how materials respond to changes in temperature.
In conclusion, specific heat is the amount of heat required to raise the temperature of a substance by 1 degree Celsius and is a significant parameter in understanding the thermal properties of materials.
Specific heat is the amount of heat required to raise the temperature of a unit mass of a substance by 1 degree Celsius. It is denoted by the symbol 'C' and is expressed in J/kg°C or cal/g°C.
Explanation
- When heat is added to a substance, its temperature increases. The specific heat of a substance determines how much heat energy is required to raise its temperature.
- Different substances have different specific heat values due to their molecular structure and composition. For example, water has a high specific heat capacity compared to metals.
- The specific heat of a substance is an important property in various fields such as thermodynamics, chemistry, and engineering as it helps in calculating the amount of heat required for temperature changes.
- The specific heat capacity of water is particularly important in nature as it helps regulate the temperature of oceans and bodies of water, which in turn influences weather patterns and climate.
Importance
- Specific heat plays a crucial role in various applications, including designing heating and cooling systems, calculating energy requirements, and understanding the behavior of substances under different temperature conditions.
- It is a fundamental property used in scientific research, industrial processes, and everyday life to determine how materials respond to changes in temperature.
In conclusion, specific heat is the amount of heat required to raise the temperature of a substance by 1 degree Celsius and is a significant parameter in understanding the thermal properties of materials.
Pi-bonds do not alter the shape, but merely shorten the- a)bond energy
- b)bond angle
- c)bond length
- d)bond frequency
Correct answer is option 'C'. Can you explain this answer?
Pi-bonds do not alter the shape, but merely shorten the
a)
bond energy
b)
bond angle
c)
bond length
d)
bond frequency
|
|
Vijay Gangle answered |
Pi bond is formed by double or triple bond which is generally have small bond lengths than sigma bond which is formed by single bonds overlapping
The eccentricity of earth's orbit is 0.0167. The ratio of its maximum speed in its orbit to its minimum speed is- a)2.507
- b)1.033
- c)8.324
- d)1.000
Correct answer is option 'B'. Can you explain this answer?
The eccentricity of earth's orbit is 0.0167. The ratio of its maximum speed in its orbit to its minimum speed is
a)
2.507
b)
1.033
c)
8.324
d)
1.000
|
|
Anagha Chavan answered |
Let e be the eccentricity of the earth's orbit about the sun and a and b its semi major and semi minor axes.
Let v1 be the maximum speed of the earth at perigee and v2 be the minimum speed at apogee.
Also let the sun be at one of the foci of the earth's orbit.
Then the distance from the Sun to perigee = (a - ae) and the distance from the sund to apogee = (a + ae)
From the principle of conservation of angular momentum
mv1(a - ae) = mv2(a + ae)
Also let the sun be at one of the foci of the earth's orbit.
Then the distance from the Sun to perigee = (a - ae) and the distance from the sund to apogee = (a + ae)
From the principle of conservation of angular momentum
mv1(a - ae) = mv2(a + ae)
Which of the following has highest first ionization potential?- a)Carbon
- b)Oxgyen
- c)Nitrogen
- d)Boron
Correct answer is option 'C'. Can you explain this answer?
Which of the following has highest first ionization potential?
a)
Carbon
b)
Oxgyen
c)
Nitrogen
d)
Boron
|
|
Reena Dhanesh Kumar answered |
THE ELECTRONIC CONFIGURATION OF NITROGEN IS 1s2 2s2 2p3 so, the nitrogen atom doesn't wish to lose/is ready to lose the half filled configuration which provides more stability.therefore high ionisation enthalpy.( half filled and completely filled orbitals stability)
Force necessary to pull a circular plate of 5 cm radius from water surface for which surface tension is 75 dynes/cm,is- a)30 dynes
- b)60 dynes
- c)750 dynes
- d)750π dynes
Correct answer is option 'D'. Can you explain this answer?
Force necessary to pull a circular plate of 5 cm radius from water surface for which surface tension is 75 dynes/cm,is
a)
30 dynes
b)
60 dynes
c)
750 dynes
d)
750π dynes

|
Rhea Joshi answered |
Surface tension = force/ length
Given: radius = 5 cm
So circumference of plate is 2*π*5 cm
Now, 75=force/2π*5 Force 750 π dyne.
Consider two rods of same length and different specific heatrs (s₁ and s₂) conductivities k₁ and k₂ and areas of cross section (A₁ and A₂) and both giving temperature T₁ and T₂ at their ends. If the rate of heat loss due to conduction is equal, then- a)K₁A₁ = K₂A₂
- b)K₂A₁ = K₁A₂
- c)K₁A₁/s₁ = K₂A₂/s₂
- d)K₂A₁/s₂ = K₂A₂/s₁
Correct answer is option 'A'. Can you explain this answer?
Consider two rods of same length and different specific heatrs (s₁ and s₂) conductivities k₁ and k₂ and areas of cross section (A₁ and A₂) and both giving temperature T₁ and T₂ at their ends. If the rate of heat loss due to conduction is equal, then
a)
K₁A₁ = K₂A₂
b)
K₂A₁ = K₁A₂
c)
K₁A₁/s₁ = K₂A₂/s₂
d)
K₂A₁/s₂ = K₂A₂/s₁
|
|
Srinidhi V answered |
Heat loss dq/dt = KA/L
As rate of heat loss is same:-
K1 A1/L = K2 A2/L ( Given, lengths are same).
Hence..
K1 A1= K2 A2
Option A is correct.
As rate of heat loss is same:-
K1 A1/L = K2 A2/L ( Given, lengths are same).
Hence..
K1 A1= K2 A2
Option A is correct.
An L.C. circuit contains 10 mH inductor and a 25 μ F capacitor. The resistance of the circuit is negligible. The energy stored in the circuit is completely magnetic at time (in milli seconds) being measured from the instant when the circuit is closed- a)1.57,4.71,7.85
- b)1.57,3.14,4.71
- c)0,1.57,4.71
- d)None of these
Correct answer is option 'A'. Can you explain this answer?
An L.C. circuit contains 10 mH inductor and a 25 μ F capacitor. The resistance of the circuit is negligible. The energy stored in the circuit is completely magnetic at time (in milli seconds) being measured from the instant when the circuit is closed
a)
1.57,4.71,7.85
b)
1.57,3.14,4.71
c)
0,1.57,4.71
d)
None of these
|
|
Rashi Chopra answered |
Therefore, t = 1.57, 4.71, 7.85 ... milli sec
The force constant of two springs are k1 and k2 respectively. Both are stretched till their potential energies are equal. The forces f1 and f2 applied on them are in the ratio- a)k1 : k2
- b)k2 : k1
- c)√k1 : √k2
- d)√k2 : √k1
Correct answer is option 'C'. Can you explain this answer?
The force constant of two springs are k1 and k2 respectively. Both are stretched till their potential energies are equal. The forces f1 and f2 applied on them are in the ratio
a)
k1 : k2
b)
k2 : k1
c)
√k1 : √k2
d)
√k2 : √k1
|
|
Maitri Roy answered |
Potential energy of spring u = 1/2 = kx2
k = force constant, x = increase in length
u1 = 1/2 k1x12
u2 = 1/2 k2x22
According to question
u1 = u2
1/2 k1x12 = 1/2 k2x22
k = force constant, x = increase in length
u1 = 1/2 k1x12
u2 = 1/2 k2x22
According to question
u1 = u2
1/2 k1x12 = 1/2 k2x22
A body of mass 60 kg is dragged with just enough force to start moving on a rough surface with coefficients of static and kinetic frictions 0.5 and 0.4 respectively. On applying the same force, what is the acceleration :- a)0.98 m/s2
- b)9.8 m/s2
- c)0.54 m/s2
- d)5.292 m/s2
Correct answer is option 'A'. Can you explain this answer?
A body of mass 60 kg is dragged with just enough force to start moving on a rough surface with coefficients of static and kinetic frictions 0.5 and 0.4 respectively. On applying the same force, what is the acceleration :
a)
0.98 m/s2
b)
9.8 m/s2
c)
0.54 m/s2
d)
5.292 m/s2

|
Ruchi Yadav answered |
Correct option (a)
Explanation:
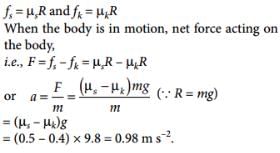
Explanation:

The resonant frequency of a circuit is f. If the capacitance is made 4 times the initial value, then the resonant frequency will becomes- a)f/2
- b)2f
- c)f
- d)f/4
Correct answer is option 'A'. Can you explain this answer?
The resonant frequency of a circuit is f. If the capacitance is made 4 times the initial value, then the resonant frequency will becomes
a)
f/2
b)
2f
c)
f
d)
f/4
|
|
Muskaan Roy answered |
Understanding Resonant Frequency
The resonant frequency of a circuit, particularly in an LC circuit, is defined by the formula:
\[ f = \frac{1}{2\pi\sqrt{LC}} \]
where:
- \( f \) is the resonant frequency,
- \( L \) is the inductance,
- \( C \) is the capacitance.
Effect of Increasing Capacitance
When the capacitance \( C \) is increased to four times its initial value, we can denote the new capacitance as \( C' = 4C \). Substituting this into the formula for resonant frequency gives:
\[ f' = \frac{1}{2\pi\sqrt{L \cdot 4C}} \]
This can be simplified as:
\[ f' = \frac{1}{2\pi\sqrt{4LC}} \]
Calculating the New Frequency
Since \( \sqrt{4} = 2 \), we can write:
\[ f' = \frac{1}{2\pi \cdot 2\sqrt{LC}} = \frac{1}{2} \cdot \frac{1}{2\pi\sqrt{LC}} \]
Thus, we have:
\[ f' = \frac{f}{2} \]
Conclusion
Therefore, increasing the capacitance by a factor of four results in the resonant frequency being halved. The correct answer is:
Option A: \( \frac{f}{2} \)
The resonant frequency of a circuit, particularly in an LC circuit, is defined by the formula:
\[ f = \frac{1}{2\pi\sqrt{LC}} \]
where:
- \( f \) is the resonant frequency,
- \( L \) is the inductance,
- \( C \) is the capacitance.
Effect of Increasing Capacitance
When the capacitance \( C \) is increased to four times its initial value, we can denote the new capacitance as \( C' = 4C \). Substituting this into the formula for resonant frequency gives:
\[ f' = \frac{1}{2\pi\sqrt{L \cdot 4C}} \]
This can be simplified as:
\[ f' = \frac{1}{2\pi\sqrt{4LC}} \]
Calculating the New Frequency
Since \( \sqrt{4} = 2 \), we can write:
\[ f' = \frac{1}{2\pi \cdot 2\sqrt{LC}} = \frac{1}{2} \cdot \frac{1}{2\pi\sqrt{LC}} \]
Thus, we have:
\[ f' = \frac{f}{2} \]
Conclusion
Therefore, increasing the capacitance by a factor of four results in the resonant frequency being halved. The correct answer is:
Option A: \( \frac{f}{2} \)
A body falling form a height of 10 m rebounds from hard floor. if it loses 20% energy in the impact, then coefficient of restitution is- a)0.89
- b)0.56
- c)0.23
- d)0.18
Correct answer is option 'A'. Can you explain this answer?
A body falling form a height of 10 m rebounds from hard floor. if it loses 20% energy in the impact, then coefficient of restitution is
a)
0.89
b)
0.56
c)
0.23
d)
0.18

|
Ishitva Sharma answered |
KE just before the collision will be in so
l
o
s
s
P
E
K
i
=
m
g
h
=
m
�
1
0
�
1
0
=
1
0
0
m
loss due to impact was % so KE will be % or
2
0
r
e
m
a
i
n
i
n
g
K
f
=
1
0
0
m
�
8
0
K
f
=
1
0
0
m
�
0
.
8
=
8
0
m
Now the velocity of strike will be
V
i
=
m
2
K
i
=
1
0
2
m
/
s
and the velocity after rebound will be
V
f
=
m
2
K
f
=
4
1
0
m
/
s
coeefificient of restitution will be
e
=
V
i
V
f
=
1
0
2
4
1
0
=
0
.
8
9
A body falls freely from rest. It covers as much distance in the last second of its motion as covered in the first three seconds. The body has fallen for a time of- a)3 s
- b)5 s
- c)7 s
- d)9 s
Correct answer is option 'B'. Can you explain this answer?
A body falls freely from rest. It covers as much distance in the last second of its motion as covered in the first three seconds. The body has fallen for a time of
a)
3 s
b)
5 s
c)
7 s
d)
9 s
|
|
Aarya Menon answered |
Concept: Kinematics of Free Fall
When an object is dropped from a certain height, it falls freely under the influence of gravity. The motion of the object is called free fall.
Some important equations of kinematics of free fall are:
1. v = u + gt
2. s = ut + (1/2)gt^2
3. v^2 = u^2 + 2gs
Where,
v = final velocity
u = initial velocity (0 in free fall)
g = acceleration due to gravity (9.8 m/s^2)
t = time taken
s = distance covered
Solution:
Given, the body falls freely from rest.
Let the time taken by the body to fall be 't' seconds.
It is given that the distance covered in the last second of motion is equal to the distance covered in the first three seconds.
So, the distance covered in the last second = distance covered in the first three seconds
Using equation (2), we can write:
s_last = u(t-1) + (1/2)g(t-1)^2
s_first_three = u(3) + (1/2)g(3)^2
Since s_last = s_first_three,
u(t-1) + (1/2)g(t-1)^2 = u(3) + (1/2)g(3)^2
Solving this equation, we get:
t = 5 seconds
Therefore, the body has fallen for a time of 5 seconds.
Hence, the correct answer is option 'B'.
When an object is dropped from a certain height, it falls freely under the influence of gravity. The motion of the object is called free fall.
Some important equations of kinematics of free fall are:
1. v = u + gt
2. s = ut + (1/2)gt^2
3. v^2 = u^2 + 2gs
Where,
v = final velocity
u = initial velocity (0 in free fall)
g = acceleration due to gravity (9.8 m/s^2)
t = time taken
s = distance covered
Solution:
Given, the body falls freely from rest.
Let the time taken by the body to fall be 't' seconds.
It is given that the distance covered in the last second of motion is equal to the distance covered in the first three seconds.
So, the distance covered in the last second = distance covered in the first three seconds
Using equation (2), we can write:
s_last = u(t-1) + (1/2)g(t-1)^2
s_first_three = u(3) + (1/2)g(3)^2
Since s_last = s_first_three,
u(t-1) + (1/2)g(t-1)^2 = u(3) + (1/2)g(3)^2
Solving this equation, we get:
t = 5 seconds
Therefore, the body has fallen for a time of 5 seconds.
Hence, the correct answer is option 'B'.
One of the refracting surfaces of a prism of refractive index √2 is silvered. The angle of the prism is equal to the critical angle of a medium of refractive index 2. A ray of light incident on the unsilvered surface passes through the prism and retraces its path after reflection at the silvered face. Then the angle of incidence on the unsilvered surface is- a)0 oC
- b)30oC
- c)45oC
- d)60 oC
Correct answer is option 'C'. Can you explain this answer?
One of the refracting surfaces of a prism of refractive index √2 is silvered. The angle of the prism is equal to the critical angle of a medium of refractive index 2. A ray of light incident on the unsilvered surface passes through the prism and retraces its path after reflection at the silvered face. Then the angle of incidence on the unsilvered surface is
a)
0 oC
b)
30oC
c)
45oC
d)
60 oC
|
|
Anuj Datta answered |
Consider the following statements about dimensions
P:Force=acceleration due to gravity x mass
Q:Electric charge=current x time
R:Magnetic flux=Electric voltage x time
The correct statements are :- a)Q,R only
- b)P,Q only
- c)P, Q and R
- d)R,P only
Correct answer is option 'C'. Can you explain this answer?
Consider the following statements about dimensions
P:Force=acceleration due to gravity x mass
Q:Electric charge=current x time
R:Magnetic flux=Electric voltage x time
The correct statements are :
P:Force=acceleration due to gravity x mass
Q:Electric charge=current x time
R:Magnetic flux=Electric voltage x time
The correct statements are :
a)
Q,R only
b)
P,Q only
c)
P, Q and R
d)
R,P only
|
|
Ritesh Chaudhary answered |
Correct answer is c
Choose the correct statement from the following. The radius of the orbit of a geostationary satellite depends upon- a)Mass of the satellite, its time period and the gravitational consatnt
- b)Mass of the satellite, mass of the earth, and the gravitational constant
- c)Mass of the earth, mass of the satellite, time period of the satellite and the gravitational consatnt
- d)Mass of the earth, time period of the satellite and the gravitational constant
Correct answer is option 'D'. Can you explain this answer?
Choose the correct statement from the following. The radius of the orbit of a geostationary satellite depends upon
a)
Mass of the satellite, its time period and the gravitational consatnt
b)
Mass of the satellite, mass of the earth, and the gravitational constant
c)
Mass of the earth, mass of the satellite, time period of the satellite and the gravitational consatnt
d)
Mass of the earth, time period of the satellite and the gravitational constant

|
Mothe Nikita Kishor Niki answered |
It depends upon the period mass of the earth according to the formula is t is equal to 2 pie under root r cube upon g m
How many atoms are contained in one mole of sucrose (C12H22O11)?- a)45 x 6.02 x 1023 atoms/mole
- b)5 x 6.62 x 1023 atoms/mole
- c)5 x 6.02 x 1023 atoms/mole
- d)None of these
Correct answer is option 'A'. Can you explain this answer?
How many atoms are contained in one mole of sucrose (C12H22O11)?
a)
45 x 6.02 x 1023 atoms/mole
b)
5 x 6.62 x 1023 atoms/mole
c)
5 x 6.02 x 1023 atoms/mole
d)
None of these
|
|
Niharika Deshpande answered |
Explanation:
To determine the number of atoms in one mole of sucrose (C12H22O11), we need to calculate the total number of atoms present in one molecule of sucrose and then multiply it by Avogadro's number (6.02 x 10^23 atoms/mol).
Step 1: Calculate the total number of atoms in one molecule of sucrose
Sucrose (C12H22O11) consists of carbon (C), hydrogen (H), and oxygen (O) atoms. The subscripts in the chemical formula represent the number of atoms of each element in one molecule of sucrose.
- Carbon (C): 12 atoms x 1 molecule = 12 atoms
- Hydrogen (H): 22 atoms x 1 molecule = 22 atoms
- Oxygen (O): 11 atoms x 1 molecule = 11 atoms
Therefore, one molecule of sucrose contains a total of 12 + 22 + 11 = 45 atoms.
Step 2: Multiply by Avogadro's number
Since one mole is defined as 6.02 x 10^23 particles (atoms, molecules, etc.), we multiply the number of atoms in one molecule of sucrose by Avogadro's number to obtain the number of atoms in one mole of sucrose.
Number of atoms in one mole of sucrose = 45 atoms/molecule x 6.02 x 10^23 atoms/mol
Simplifying this expression, we get:
Number of atoms in one mole of sucrose = 45 x 6.02 x 10^23 atoms/mole
Therefore, the correct answer is option 'A': 45 x 6.02 x 10^23 atoms/mole.
To determine the number of atoms in one mole of sucrose (C12H22O11), we need to calculate the total number of atoms present in one molecule of sucrose and then multiply it by Avogadro's number (6.02 x 10^23 atoms/mol).
Step 1: Calculate the total number of atoms in one molecule of sucrose
Sucrose (C12H22O11) consists of carbon (C), hydrogen (H), and oxygen (O) atoms. The subscripts in the chemical formula represent the number of atoms of each element in one molecule of sucrose.
- Carbon (C): 12 atoms x 1 molecule = 12 atoms
- Hydrogen (H): 22 atoms x 1 molecule = 22 atoms
- Oxygen (O): 11 atoms x 1 molecule = 11 atoms
Therefore, one molecule of sucrose contains a total of 12 + 22 + 11 = 45 atoms.
Step 2: Multiply by Avogadro's number
Since one mole is defined as 6.02 x 10^23 particles (atoms, molecules, etc.), we multiply the number of atoms in one molecule of sucrose by Avogadro's number to obtain the number of atoms in one mole of sucrose.
Number of atoms in one mole of sucrose = 45 atoms/molecule x 6.02 x 10^23 atoms/mol
Simplifying this expression, we get:
Number of atoms in one mole of sucrose = 45 x 6.02 x 10^23 atoms/mole
Therefore, the correct answer is option 'A': 45 x 6.02 x 10^23 atoms/mole.
An example of unstriated muscles which are entirely involuntary are located- a) In the diaphragm
- b) In the eyelid
- c) At the pylorus
- d) At the base of external ear
Correct answer is option 'C'. Can you explain this answer?
a)
In the diaphragm
b)
In the eyelid
c)
At the pylorus
d)
At the base of external ear
|
|
Mira Sharma answered |
A lymph duct is a great lymphaticvessel that empties lymph into one of the subclavian veins. There are twolymph ducts in the body—the rightlymphatic duct and the thoracic duct. The right lymphatic duct drains lymphfrom the right upper limb, right side of thorax and right halves of head and neck. OPTION (C) IS CORRECT ANSWER.
A ray of light falls on a transparent glass slab of refractive index 1.62. If the reflected ray and the refracted ray are mutually perpendicular, the angle of incidence is- a)tan-1(1.62)
- b)tan-1(1/1.62)
- c)tan-1(1.33)
- d)tan-1(1/1.33)
Correct answer is option 'A'. Can you explain this answer?
A ray of light falls on a transparent glass slab of refractive index 1.62. If the reflected ray and the refracted ray are mutually perpendicular, the angle of incidence is
a)
tan-1(1.62)
b)
tan-1(1/1.62)
c)
tan-1(1.33)
d)
tan-1(1/1.33)
|
|
Maulik Menon answered |
Explanation:
When a ray of light falls on a transparent glass slab, it undergoes reflection and refraction. The angle of incidence is the angle between the incident ray and the normal to the surface of the glass slab. The angle of reflection is the angle between the reflected ray and the normal, and the angle of refraction is the angle between the refracted ray and the normal.
In this case, the reflected ray and the refracted ray are mutually perpendicular. This means that the angle of reflection is 90 degrees. Let's assume that the angle of incidence is θ.
Using the laws of reflection and refraction:
1. Law of reflection: The angle of incidence is equal to the angle of reflection. Therefore, the angle of reflection is θ.
2. Snell's law: The ratio of the sine of the angle of incidence to the sine of the angle of refraction is equal to the ratio of the refractive indices of the two media. In this case, the refractive index of the glass slab is 1.62. Therefore, we can write:
sin(θ)/sin(90 degrees) = 1/1.62
sin(θ) = 1/1.62
Using the trigonometric identity:
sin^2(θ) + cos^2(θ) = 1
Substituting the value of sin(θ) from Snell's law:
(1/1.62)^2 + cos^2(θ) = 1
1/2.6244 + cos^2(θ) = 1
cos^2(θ) = 1 - 1/2.6244
cos^2(θ) = 0.6177
cos(θ) = sqrt(0.6177)
cos(θ) = 0.7855
Using the trigonometric identity:
tan^2(θ) + 1 = sec^2(θ)
Substituting the value of cos(θ) from the previous step:
tan^2(θ) + 1 = 1/0.6177
tan^2(θ) = 1/0.6177 - 1
tan^2(θ) = 0.6177/0.6177 - 1
tan^2(θ) = 1 - 0.6177/0.6177
tan^2(θ) = 0.3823/0.6177
tan^2(θ) = 0.6189
tan(θ) = sqrt(0.6189)
tan(θ) = 0.7869
Therefore, the angle of incidence is:
θ = tan^(-1)(0.7869)
Hence, the correct answer is option 'A': tan^(-1)(1.62).
When a ray of light falls on a transparent glass slab, it undergoes reflection and refraction. The angle of incidence is the angle between the incident ray and the normal to the surface of the glass slab. The angle of reflection is the angle between the reflected ray and the normal, and the angle of refraction is the angle between the refracted ray and the normal.
In this case, the reflected ray and the refracted ray are mutually perpendicular. This means that the angle of reflection is 90 degrees. Let's assume that the angle of incidence is θ.
Using the laws of reflection and refraction:
1. Law of reflection: The angle of incidence is equal to the angle of reflection. Therefore, the angle of reflection is θ.
2. Snell's law: The ratio of the sine of the angle of incidence to the sine of the angle of refraction is equal to the ratio of the refractive indices of the two media. In this case, the refractive index of the glass slab is 1.62. Therefore, we can write:
sin(θ)/sin(90 degrees) = 1/1.62
sin(θ) = 1/1.62
Using the trigonometric identity:
sin^2(θ) + cos^2(θ) = 1
Substituting the value of sin(θ) from Snell's law:
(1/1.62)^2 + cos^2(θ) = 1
1/2.6244 + cos^2(θ) = 1
cos^2(θ) = 1 - 1/2.6244
cos^2(θ) = 0.6177
cos(θ) = sqrt(0.6177)
cos(θ) = 0.7855
Using the trigonometric identity:
tan^2(θ) + 1 = sec^2(θ)
Substituting the value of cos(θ) from the previous step:
tan^2(θ) + 1 = 1/0.6177
tan^2(θ) = 1/0.6177 - 1
tan^2(θ) = 0.6177/0.6177 - 1
tan^2(θ) = 1 - 0.6177/0.6177
tan^2(θ) = 0.3823/0.6177
tan^2(θ) = 0.6189
tan(θ) = sqrt(0.6189)
tan(θ) = 0.7869
Therefore, the angle of incidence is:
θ = tan^(-1)(0.7869)
Hence, the correct answer is option 'A': tan^(-1)(1.62).
Chapter doubts & questions for Medical - SRMJEEE Subject Wise & Full Length Mock Tests 2026 2025 is part of JEE exam preparation. The chapters have been prepared according to the JEE exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for JEE 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Medical - SRMJEEE Subject Wise & Full Length Mock Tests 2026 in English & Hindi are available as part of JEE exam.
Download more important topics, notes, lectures and mock test series for JEE Exam by signing up for free.

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up
within 7 days!
within 7 days!
Takes less than 10 seconds to signup