All Exams >
MCAT >
MCAT Chemical and Physical Foundations >
All Questions
All questions of Electronic Structure (PHY, GC) for MCAT Exam
The energy of the incident photon is 20 eV and the work function of the photosensitive metal is 10 eV. What is the stopping potential?- a)30 V
- b)5 V
- c)10 V
- d)15 V
Correct answer is option 'C'. Can you explain this answer?
The energy of the incident photon is 20 eV and the work function of the photosensitive metal is 10 eV. What is the stopping potential?
a)
30 V
b)
5 V
c)
10 V
d)
15 V
|
|
Krishna Iyer answered |
Stopping potential (Vo) is given by
Vo=W/q where W is the work function and q are the charge of an electron.
Given W=20eV−10eV=10eV. Also, q=e
Hence, Vo=(10eV)/e=10V
Vo=W/q where W is the work function and q are the charge of an electron.
Given W=20eV−10eV=10eV. Also, q=e
Hence, Vo=(10eV)/e=10V
For Balmer series,the initial state n1 is :- a)4
- b)2
- c)3
- d)1
Correct answer is option 'B'. Can you explain this answer?
For Balmer series,the initial state n1 is :
a)
4
b)
2
c)
3
d)
1
|
|
Pooja Shah answered |
The Balmer series just sets n1 = 2, which means the value of the principal quantum number (n) is two for the transitions being considered. Balmer’s formula can therefore be written:
1/λ = RH ((1/22) − (1 / n22))
The energy associated with the transition of an electron from the n=1 state to the n=3 state of H atoms is:- a)+1.74 x 10-17 Joules.
- b)-1.94 x 10-18 Joules.
- c)+1.94 x 10-18 Joules.
- d)-1.74 x 10-17 Joules.
Correct answer is option 'C'. Can you explain this answer?
The energy associated with the transition of an electron from the n=1 state to the n=3 state of H atoms is:
a)
+1.74 x 10-17 Joules.
b)
-1.94 x 10-18 Joules.
c)
+1.94 x 10-18 Joules.
d)
-1.74 x 10-17 Joules.
|
|
Lavanya Menon answered |
The formula to calculate the excitation energy is 13.6Z2(1/n12-1/n22), but this gives value in eV. To convert it in Joules we divide it by 6.24×1018 Here, Z=1,n1=1,n2=3 Putting these values in above formula we have, [13.6×1(1-1/9)]/6.24×1018 =(13.6×8×10-18)/(9×6.24) =1.94×10-18 Hence, the correct answer is C.
In the absorption spectrum, the wavelengths which are absorbed, are missing and they appear as:- a)Bright lines
- b)Light bands
- c)Bright bands
- d)Dark lines
Correct answer is option 'D'. Can you explain this answer?
In the absorption spectrum, the wavelengths which are absorbed, are missing and they appear as:
a)
Bright lines
b)
Light bands
c)
Bright bands
d)
Dark lines
|
|
Riya Banerjee answered |
Light not absorbed by the sample will, as before, be separated (dispersed) into its component wavelengths (colors) by the prism. The appearance of the spectrum will resemble that obtained without the sample in place, with the exception that those wavelengths which have been absorbed are missing, and will appear as dark lines within the spectrum of colors. If a piece of the photographic film is used instead of the card, the absorption spectrum can be recorded.
Photons of energy 6 eV are incident on a potassium surface of a work function 2.1 eV. What is the stopping potential?- a)-3.9V
- b)-8.1V
- c)-5V
- d)-1.9V
Correct answer is 'A'. Can you explain this answer?
Photons of energy 6 eV are incident on a potassium surface of a work function 2.1 eV. What is the stopping potential?
a)
-3.9V
b)
-8.1V
c)
-5V
d)
-1.9V
|
|
Priyanka Sharma answered |
From photo-electric equation, eV0= E−φ
eV0=(6−2.1)eV
V0= 3.9 V
stopping potential is a negative potential to stop e- at saturated current .
eV0=(6−2.1)eV
V0= 3.9 V
stopping potential is a negative potential to stop e- at saturated current .
Light of frequency 1.5 times the threshold frequency is incident on a photosensitive material .If the frequency is halved and the intensity is doubled, the photoelectric current becomes- a)Doubled
- b)Quadrupled
- c)Halved
- d)zero
Correct answer is option 'D'. Can you explain this answer?
Light of frequency 1.5 times the threshold frequency is incident on a photosensitive material .If the frequency is halved and the intensity is doubled, the photoelectric current becomes
a)
Doubled
b)
Quadrupled
c)
Halved
d)
zero

|
Ayush Joshi answered |
If the frequency is halved and intensity is doubled, the frequency of incident light will become 15/2 = 0.75 times the threshold frequency. So, as ν<νo Hence, photoelectric current will be zero.
Can you explain the answer of this question below:Light from a bulb is falling on a wooden table but no photo electrons are emitted as
- A:
Work function of wood is less
- B:
Work function of wood is more
- C:
It depends on the frequency
- D:
It is independent of work function
The answer is b.
Light from a bulb is falling on a wooden table but no photo electrons are emitted as
Work function of wood is less
Work function of wood is more
It depends on the frequency
It is independent of work function
|
|
Rishika Patel answered |
Explanation:
When light falls on a metal surface, electrons may be emitted from the metal surface. This phenomenon is known as the photoelectric effect. The electrons emitted from the metal surface are called photoelectrons.
The photoelectric effect can be explained by considering that light is made up of photons. Each photon has a certain amount of energy, given by its frequency. When a photon strikes a metal surface, its energy can be transferred to an electron in the metal. If the energy of the photon is greater than the work function of the metal, the electron can be emitted from the metal surface.
In the case of a wooden table, the work function of the wood is more than the energy of the photons of the light falling on it. Therefore, no photoelectrons are emitted. This is because the energy of the photons is not enough to overcome the work function of the wood.
Key Points:
- The photoelectric effect is the emission of electrons from a metal surface when light falls on it.
- The energy of a photon is given by its frequency.
- If the energy of a photon is greater than the work function of the metal, electrons can be emitted from the metal surface.
- In the case of a wooden table, the work function of the wood is more than the energy of the photons of the light falling on it, so no photoelectrons are emitted.
When light falls on a metal surface, electrons may be emitted from the metal surface. This phenomenon is known as the photoelectric effect. The electrons emitted from the metal surface are called photoelectrons.
The photoelectric effect can be explained by considering that light is made up of photons. Each photon has a certain amount of energy, given by its frequency. When a photon strikes a metal surface, its energy can be transferred to an electron in the metal. If the energy of the photon is greater than the work function of the metal, the electron can be emitted from the metal surface.
In the case of a wooden table, the work function of the wood is more than the energy of the photons of the light falling on it. Therefore, no photoelectrons are emitted. This is because the energy of the photons is not enough to overcome the work function of the wood.
Key Points:
- The photoelectric effect is the emission of electrons from a metal surface when light falls on it.
- The energy of a photon is given by its frequency.
- If the energy of a photon is greater than the work function of the metal, electrons can be emitted from the metal surface.
- In the case of a wooden table, the work function of the wood is more than the energy of the photons of the light falling on it, so no photoelectrons are emitted.
A photo-sensitive material would emit electrons, if excited by photons beyond a threshold. To overcome the threshold, one would increase the:- a) voltage applied to the light source
- b) intensity of light
- c) wavelength of light
- d) frequency of light
Correct answer is option 'D'. Can you explain this answer?
A photo-sensitive material would emit electrons, if excited by photons beyond a threshold. To overcome the threshold, one would increase the:
a)
voltage applied to the light source
b)
intensity of light
c)
wavelength of light
d)
frequency of light
|
|
Rohan Singh answered |
The emission of photoelectron takes place only, when the frequency of the incident light is above a certain critical value, characteristic of that metal. The critical value of frequency is known as the threshold frequency for the metal of the emitting electrode.
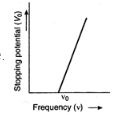
Suppose that when light of certain frequency is incident over a metal surface, the photo- electrons are emitted. To take photoelectric current zero, a particular value of stopping potential will be needed. If we go on reducing the frequency of incident light, the value of stopping potential will also go on decreasing. At certain value of frequency v0, the photoelectric current will become zero, even when no retarding potential is applied. This frequency vq corresponds to the threshold for the metal surface. The emission of photoelectrons does not take place, till frequency of incident light is below this value.

How much energy is needed to ionize a hydrogen atom if electron is present in n=1 orbit?- a)13.6 eV
- b)10.2 eV
- c)3.4eV
- d)1 eV
Correct answer is option 'A'. Can you explain this answer?
How much energy is needed to ionize a hydrogen atom if electron is present in n=1 orbit?
a)
13.6 eV
b)
10.2 eV
c)
3.4eV
d)
1 eV
|
|
Pooja Shah answered |
For hydrogen Z=1,
and n is given as 1,
Then
E= -13.6 × n power 2/ Z.
E= - 13.6 × 1 power 2/ 1.
E= -13.6e.v.
and n is given as 1,
Then
E= -13.6 × n power 2/ Z.
E= - 13.6 × 1 power 2/ 1.
E= -13.6e.v.
Sodium surface is illuminated by ultraviolet and visible radiation successively and the stopping potential determined. The stopping potential is- a)Greater with visible light
- b)Equal in both cases
- c)Greater with ultraviolet light
- d)Infinite in both cases
Correct answer is 'C'. Can you explain this answer?
Sodium surface is illuminated by ultraviolet and visible radiation successively and the stopping potential determined. The stopping potential is
a)
Greater with visible light
b)
Equal in both cases
c)
Greater with ultraviolet light
d)
Infinite in both cases
|
|
Riya Banerjee answered |
λ for U.V is less than λ for visible light
ν for U.V is greater than ν for visible light
∴ potential is greater for U.V light.
as K.Eα1/λ
ν for U.V is greater than ν for visible light
∴ potential is greater for U.V light.
as K.Eα1/λ
The total energy of an electron in the nth orbit of a hydrogen atom is given by the formula
En = -13.6 eV/n2. What does the negative energy for an electron indicate?- a)Energy of electron in an atom is lower than energy of an electron far away from nucleus
- b)The electron has a negative charge.
- c)The electron is far away from nucleus
- d)Electrons have both wave and particle-like properties.
Correct answer is option 'A'. Can you explain this answer?
The total energy of an electron in the nth orbit of a hydrogen atom is given by the formula
En = -13.6 eV/n2. What does the negative energy for an electron indicate?
En = -13.6 eV/n2. What does the negative energy for an electron indicate?
a)
Energy of electron in an atom is lower than energy of an electron far away from nucleus
b)
The electron has a negative charge.
c)
The electron is far away from nucleus
d)
Electrons have both wave and particle-like properties.
|
|
Gaurav Kumar answered |
The negative sign means that the energy of the electron in the atom is lower than the energy of a free electron at rest. A free electron at rest is an electron that is infinitely far away from the nucleus and is assigned the energy value zero.
Calculate the wavelength of light that corresponds to the radiation that is given off during the transition of an electron from the n = 5 to n = 2 state of the hydrogen atom.- a)434 nm
- b)275 nm
- c)305 nm
- d)183 nm
Correct answer is option 'A'. Can you explain this answer?
Calculate the wavelength of light that corresponds to the radiation that is given off during the transition of an electron from the n = 5 to n = 2 state of the hydrogen atom.
a)
434 nm
b)
275 nm
c)
305 nm
d)
183 nm
|
|
Hansa Sharma answered |
1/wavelength =RH x z2 x (1/22-1/52) =109677 x 1 x (1/4-1/25) =109677 x 21/100 =2303.2m wavelength=1/2303.2m =1/2303.2 x 107nm =434.1nm~434nm
Zeeman effect is the splitting of spectral line in presence of:- a)electricity
- b)magnetic effect
- c)molecule
- d)electric field
Correct answer is option 'B'. Can you explain this answer?
Zeeman effect is the splitting of spectral line in presence of:
a)
electricity
b)
magnetic effect
c)
molecule
d)
electric field
|
|
Lavanya Menon answered |
The Zeeman effect is the splitting of the spectral lines of an atom in the presence of a strong magnetic field. The effect is due to the distortion of the electron orbitals because of the magnetic field. The (normal) Zeeman effect can be understood classically, as Lorentz predicted.
Light of wavelength 4000 Â is incident on a metal of work function 3.2 x 10-19 J. What is the maximum kinetic energy of the emitted electron?
- a)2.2 x 10-19 J
- b)1.75 x 10-19 J
- c)0.75 x 10-19 J
- d)1.1 x 10-19 J
Correct answer is option 'B'. Can you explain this answer?
Light of wavelength 4000 Â is incident on a metal of work function 3.2 x 10-19 J. What is the maximum kinetic energy of the emitted electron?
a)
2.2 x 10-19 J
b)
1.75 x 10-19 J
c)
0.75 x 10-19 J
d)
1.1 x 10-19 J
|
|
Om Desai answered |
Given,
λ= 4000 Å ;W=3.2× 10-19 j
as we know that
K.E = (hc/ λ) - W
= (6.6× 10-34 × 3 × 108/ 4000×10-10 )- 3.20× 10-19
= 1.75 × 10-19
λ= 4000 Å ;W=3.2× 10-19 j
as we know that
K.E = (hc/ λ) - W
= (6.6× 10-34 × 3 × 108/ 4000×10-10 )- 3.20× 10-19
= 1.75 × 10-19
Direction (Q. Nos. 11 and 12) This section contains a paragraph, wach describing theory, experiments, data etc. three Questions related to paragraph have been given.Each question have only one correct answer among the four given ptions (a),(b),(c),(d)Photoelectric effect can be expressed in terms of the following graph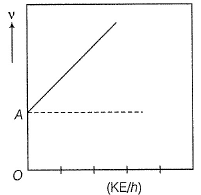
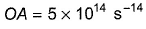 Q. What is work function for this photoelectric emission of electrons?
Q. What is work function for this photoelectric emission of electrons?- a)199.262 kJ mol-1
- b)199.262 J mol-1
- c)3.3 kJ mol-1
- d)3.3 x 10-19 kJ mol-1
Correct answer is option 'A'. Can you explain this answer?
Direction (Q. Nos. 11 and 12) This section contains a paragraph, wach describing theory, experiments, data etc. three Questions related to paragraph have been given.Each question have only one correct answer among the four given ptions (a),(b),(c),(d)
Photoelectric effect can be expressed in terms of the following graph
Q. What is work function for this photoelectric emission of electrons?
a)
199.262 kJ mol-1
b)
199.262 J mol-1
c)
3.3 kJ mol-1
d)
3.3 x 10-19 kJ mol-1
|
|
Gaurav Kumar answered |
Photoelectric effect is represented by
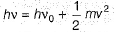
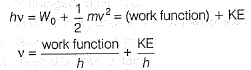

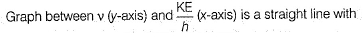
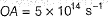
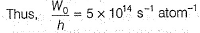
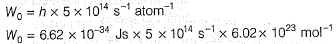
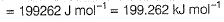
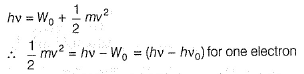
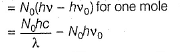
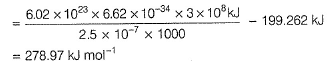
Which of the following subatomic particles is responsible for the spectrum of radiation emitted by an element or compound?- a)neutron
- b)electron
- c)proton
- d)photon
Correct answer is option 'B'. Can you explain this answer?
Which of the following subatomic particles is responsible for the spectrum of radiation emitted by an element or compound?
a)
neutron
b)
electron
c)
proton
d)
photon
|
|
Om Desai answered |
Any radiation is emitted in the quantized form as photons. These photons are actually generated when an electron changes its energy level. This can happen if the atom gets the required work function. For e.g. The emission of X Ray. When the cathode ray hits the nuclei of heavier metals, two cases may occur -
1)Nuclei absorbs the electron from K shell and this process results in radiation of energy in form of X Ray photons.
2)Auger Effect: Sometimes during the collision, an electron from main atom can also be emitted, releasing energy in form of X Ray photons. So, Now it should be more clear that while photons are the product, the real culprit behind the crime scene. So correct option is B These things can be understood easily if you know the basics of Particle Physics.
1)Nuclei absorbs the electron from K shell and this process results in radiation of energy in form of X Ray photons.
2)Auger Effect: Sometimes during the collision, an electron from main atom can also be emitted, releasing energy in form of X Ray photons. So, Now it should be more clear that while photons are the product, the real culprit behind the crime scene. So correct option is B These things can be understood easily if you know the basics of Particle Physics.
An electron falls from one energy level to another; it releases a certain amount of light with a frequency of 5.100 x 1014 Hz. What energy is associated with this electron?
a)1.3 13x 1022 Jb)3.379 x 10-20 kJc)33.8 x 10-20 Jd)1.79 0x 10-10kJCorrect answer is option 'C'. Can you explain this answer?
|
|
Preeti Iyer answered |
E = h.v
= 6.63 x 10^-34 x 5.1 x 10^14
= 33.8 x 10^-20 J
Which of the following is not true about Bohr’s model of the atom?- a)Electrons behave like waves and particles simultaneously.
- b)Electrons revolve in discrete, quantized orbits only.
- c)mvr = nh/ 2π
- d)Atoms radiate discrete Electromagnetic energies only.
Correct answer is option 'A'. Can you explain this answer?
Which of the following is not true about Bohr’s model of the atom?
a)
Electrons behave like waves and particles simultaneously.
b)
Electrons revolve in discrete, quantized orbits only.
c)
mvr = nh/ 2π
d)
Atoms radiate discrete Electromagnetic energies only.
|
|
Suresh Reddy answered |
It was De broglie who proposed the dual nature of matter and that of electrons. Davisson and germer went on to experimentally verify his theory, regarding nature of electrons(they have both particle and wave nature).
Heisenberg further introduced the uncertainty principle, related to dual nature of matter.
Bohr never putforth any such ideals and considered electron as a particle.
Heisenberg further introduced the uncertainty principle, related to dual nature of matter.
Bohr never putforth any such ideals and considered electron as a particle.
By use of a suitable filter, the green mercury emission line can be isolated. This line has a wavelength of 546.1 nm. What is the frequency? [1 Hz = 1 s-1]- a)1.820 x 104 Hz
- b)5.461 x 10-7 Hz
- c)1.820 x 10-13 Hz
- d)0.0054 x 1017 Hz
Correct answer is option 'D'. Can you explain this answer?
By use of a suitable filter, the green mercury emission line can be isolated. This line has a wavelength of 546.1 nm. What is the frequency? [1 Hz = 1 s-1]
a)
1.820 x 104 Hz
b)
5.461 x 10-7 Hz
c)
1.820 x 10-13 Hz
d)
0.0054 x 1017 Hz
|
|
Anjana Sharma answered |
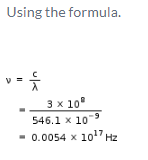
Light of two different frequencies whose photons have energies of 1eV and 2.5 eV respectively successively illuminate a metal of work function 0.5eV. The ratio of maximum speed of emitted electrons is- a)1:5
- b)1:4
- c)1:3
- d)1:2
Correct answer is option 'D'. Can you explain this answer?
Light of two different frequencies whose photons have energies of 1eV and 2.5 eV respectively successively illuminate a metal of work function 0.5eV. The ratio of maximum speed of emitted electrons is
a)
1:5
b)
1:4
c)
1:3
d)
1:2
|
|
Shreya Gupta answered |
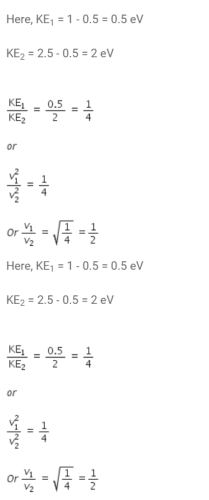
The position of both, an electron and a helium atom is known within 1.0 mm. Further more the momentum of the electron is known within 5.0 x 10-26 kg ms-1. The minimum uncertainty in the measurement of the momentum of the helium atom is- a)50.0 kg ms-1
- b)80.0 kg ms-1
- c)80.0 x 10-26 kg ms-1
- d)5.0 x 10-26 kg ms-1
Correct answer is option 'D'. Can you explain this answer?
The position of both, an electron and a helium atom is known within 1.0 mm. Further more the momentum of the electron is known within 5.0 x 10-26 kg ms-1. The minimum uncertainty in the measurement of the momentum of the helium atom is
a)
50.0 kg ms-1
b)
80.0 kg ms-1
c)
80.0 x 10-26 kg ms-1
d)
5.0 x 10-26 kg ms-1

|
Nabanita Singh answered |
Given,
Position of both an electron and a Helium atom = 1 nm
The momentum of an electron =
Uncertainty principle: It is defined as the position and the momentum both can not be determined simultaneously.
According to the Uncertainty principle,

h = Planck`s constant
When the position of an electron and helium atom is the same and the momentum of an electron is known then the momentum of the helium atom is equal to the momentum of an electron.
Therefore, the momentum of the Helium atom is 5.0 x 10-26 kg ms-1
When is hydrogen stable?- a)When the electron jumps to higher energy levels
- b)When an electric field is introduced
- c)When a magnetic field is introduced
- d)When the electron is at its ground state
Correct answer is option 'D'. Can you explain this answer?
When is hydrogen stable?
a)
When the electron jumps to higher energy levels
b)
When an electric field is introduced
c)
When a magnetic field is introduced
d)
When the electron is at its ground state
|
|
Vivek Khatri answered |
The hydrogen atom is stable when the electron rests at the ground level of energy, i.e. when the principal quantum number, n = 1. In other words, when the electron is revolving in the first orbit around the nucleus, the hydrogen atom is stable.
Can you explain the answer of this question below:The threshold frequency v0 for a metal is 7.0 x 1014 s-1. Radiation of frequency v = 1.0 x 1015 s-1 hits the metal. Kinetic energy of the emitted electron is
- A:
2.0 x 10-18 J
- B:
1.60 x 10-17 J
- C:
1.60 x 10-19 J
- D:
2.0 x 10-19 J
The answer is d.
The threshold frequency v0 for a metal is 7.0 x 1014 s-1. Radiation of frequency v = 1.0 x 1015 s-1 hits the metal. Kinetic energy of the emitted electron is
2.0 x 10-18 J
1.60 x 10-17 J
1.60 x 10-19 J
2.0 x 10-19 J
|
|
Suresh Reddy answered |
By photoelectric effect
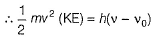
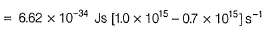
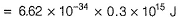
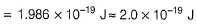
EM spectrum shows all forms of radiation except one of the followings:- a)X-rays
- b)Infra red
- c)Gamma Rays
- d)Beta rays
Correct answer is option 'D'. Can you explain this answer?
EM spectrum shows all forms of radiation except one of the followings:
a)
X-rays
b)
Infra red
c)
Gamma Rays
d)
Beta rays
|
|
Rohit Shah answered |
Uses of beta radiation. Beta radiation is used for tracers and monitoring the thickness of materials. Doctors may use radioactive chemicals called tracers for medical imaging. Certain chemicals concentrate in different damaged or diseased parts of the body, and the radiation concentrates with it.
Photoelectric effect can be expressed in terms of the following graph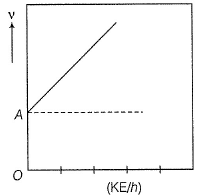
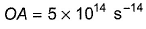 Q. Kinetic energy imparted to the moving electron at a wavelength of 2.5 x 10-7 m is
Q. Kinetic energy imparted to the moving electron at a wavelength of 2.5 x 10-7 m is- a)-278.97 kJ mol-1
- b)278.97 kJmol-1
- c)168.23 kJmol-1
- d)-168.23 kJ mol-1
Correct answer is option 'B'. Can you explain this answer?
Photoelectric effect can be expressed in terms of the following graph
Q. Kinetic energy imparted to the moving electron at a wavelength of 2.5 x 10-7 m is
a)
-278.97 kJ mol-1
b)
278.97 kJmol-1
c)
168.23 kJmol-1
d)
-168.23 kJ mol-1

|
Pioneer Academy answered |
Photoelectric effect is represented by
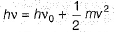


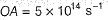
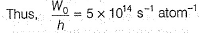
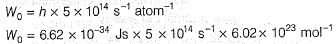
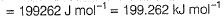
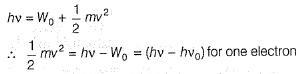
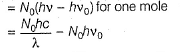
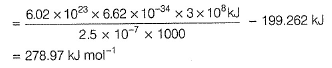
Statement I : It is impossible to determine position and momentum of the moving electron simultaneously with same accuracy.Statement II : The path of an electron in an atom is clearly defined.- a)Both Statement I and Statement II are correct and Statement II is the correct explanation of Statement I
- b)Both Statement I and Statement II are correct and Statement II is not the correct explanation of Statement I
- c)Statement I is correct but Statement II is incorrect
- d)Statement II is correct but Statement I is incorrect
Correct answer is option 'C'. Can you explain this answer?
Statement I : It is impossible to determine position and momentum of the moving electron simultaneously with same accuracy.
Statement II : The path of an electron in an atom is clearly defined.
a)
Both Statement I and Statement II are correct and Statement II is the correct explanation of Statement I
b)
Both Statement I and Statement II are correct and Statement II is not the correct explanation of Statement I
c)
Statement I is correct but Statement II is incorrect
d)
Statement II is correct but Statement I is incorrect
|
|
Neha Joshi answered |
Statement 1 is uncertainty principle, according to which we can't locate a moving electron accurately at any instant, which implies there must me certain uncertainty in its path, as path basically gives us the position of electron at various instances.
Which of the following is not a type of electromagnetic radiation?- a)x-rays
- b)sound waves
- c)ultraviolet radiation
- d)red light
Correct answer is option 'B'. Can you explain this answer?
Which of the following is not a type of electromagnetic radiation?
a)
x-rays
b)
sound waves
c)
ultraviolet radiation
d)
red light
|
|
Jithin Saini answered |
Electromagnetic Radiation
Electromagnetic radiation is a form of energy that is all around us. It can be classified into various types based on their wavelengths and frequencies.
X-rays, Ultraviolet Radiation, and Red Light
X-rays, ultraviolet radiation, and red light are all examples of electromagnetic radiation. X-rays have very high energy and short wavelengths, making them suitable for medical imaging and security screening. Ultraviolet radiation has slightly longer wavelengths than x-rays and is responsible for causing sunburns and skin damage. Red light, on the other hand, has longer wavelengths and lower energy compared to x-rays and ultraviolet radiation. It is part of the visible light spectrum and is the lowest energy form of electromagnetic radiation that can be detected by the human eye.
Sound Waves
Sound waves are not a form of electromagnetic radiation. They are mechanical waves that require a medium, such as air or water, to travel through. Sound waves are produced by vibrations and can be heard by our ears. Unlike electromagnetic radiation, sound waves cannot travel through a vacuum because they rely on the vibration of particles in a medium to propagate. Sound waves have frequencies and amplitudes but are not considered a type of electromagnetic radiation.
Electromagnetic radiation is a form of energy that is all around us. It can be classified into various types based on their wavelengths and frequencies.
X-rays, Ultraviolet Radiation, and Red Light
X-rays, ultraviolet radiation, and red light are all examples of electromagnetic radiation. X-rays have very high energy and short wavelengths, making them suitable for medical imaging and security screening. Ultraviolet radiation has slightly longer wavelengths than x-rays and is responsible for causing sunburns and skin damage. Red light, on the other hand, has longer wavelengths and lower energy compared to x-rays and ultraviolet radiation. It is part of the visible light spectrum and is the lowest energy form of electromagnetic radiation that can be detected by the human eye.
Sound Waves
Sound waves are not a form of electromagnetic radiation. They are mechanical waves that require a medium, such as air or water, to travel through. Sound waves are produced by vibrations and can be heard by our ears. Unlike electromagnetic radiation, sound waves cannot travel through a vacuum because they rely on the vibration of particles in a medium to propagate. Sound waves have frequencies and amplitudes but are not considered a type of electromagnetic radiation.
The photoelectric threshold frequency of a metal is f0. When light of frequency 4f0is incident on the metal, the maximum K.E. of the emitted electron is- a)2 hf0
- b)4 hf0
- c)3 hf0
- d)hf0/ 2
Correct answer is option 'C'. Can you explain this answer?
The photoelectric threshold frequency of a metal is f0. When light of frequency 4f0is incident on the metal, the maximum K.E. of the emitted electron is
a)
2 hf0
b)
4 hf0
c)
3 hf0
d)
hf0/ 2
|
|
Hansa Sharma answered |
The maximum kinetic energy of the emitted electrons is given by
Kmax=hυ−ϕ0=h(4υ)−h(υ)=3hυ
Kmax=hυ−ϕ0=h(4υ)−h(υ)=3hυ
For Paschen series, the initial state n1 is :- a)2
- b)4
- c)3
- d)1
Correct answer is option 'C'. Can you explain this answer?
For Paschen series, the initial state n1 is :
a)
2
b)
4
c)
3
d)
1
|
|
Riya Banerjee answered |
Paschen series from n = 4, 5, 6, 7……to n = 3
If you know that an electron is inside a 25 cm wide X-ray radiography machine what is the error on the most precise measurement that you could make of its momentum? Planck’s constant is 6.6 X 10−34 m2, kg/s.- a)4 X 10−34 kg x m/s
- b)2 X 10−34 kg x m/s
- c)2 X 10−14 kg x m/s
- d)4 X 10−14 kg x m/s
Correct answer is option 'B'. Can you explain this answer?
If you know that an electron is inside a 25 cm wide X-ray radiography machine what is the error on the most precise measurement that you could make of its momentum? Planck’s constant is 6.6 X 10−34 m2, kg/s.
a)
4 X 10−34 kg x m/s
b)
2 X 10−34 kg x m/s
c)
2 X 10−14 kg x m/s
d)
4 X 10−14 kg x m/s
|
|
Ayesha Joshi answered |
This is an application of Heisenberg’s uncertainty principle.
The uncertainty of the position is determined by knowing that the electron is somewhere inside the 25 cm wide machine, so x = 0.25 m.
The minimum uncertainty is met when the inequality in the Heisenberg uncertainty inequality becomes an equation: xp > h/4pi. Solving the equation for p, we have p = 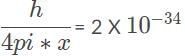 kg x m/s, the most precise measurement for the electron’s momentum when it is inside the radiography machine.
kg x m/s, the most precise measurement for the electron’s momentum when it is inside the radiography machine.
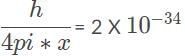 kg x m/s, the most precise measurement for the electron’s momentum when it is inside the radiography machine.
kg x m/s, the most precise measurement for the electron’s momentum when it is inside the radiography machine.Photons of the same wavelength strike four metal targets in a vacuum. Electrons are measured leaving the surfaces at various speeds. Based on the velocities of the ejected electrons, which metal has the largest work function?- a)Metal 2, with ejection velocity of 2990 m/s
- b)Metal 1, with ejection velocity of 299 m/s
- c)Metal 3, with ejection velocity of 0.0000001c
- d)Metal 4, with ejection velocity of 0.000001c
Correct answer is option 'C'. Can you explain this answer?
Photons of the same wavelength strike four metal targets in a vacuum. Electrons are measured leaving the surfaces at various speeds. Based on the velocities of the ejected electrons, which metal has the largest work function?
a)
Metal 2, with ejection velocity of 2990 m/s
b)
Metal 1, with ejection velocity of 299 m/s
c)
Metal 3, with ejection velocity of 0.0000001c
d)
Metal 4, with ejection velocity of 0.000001c
|
|
Ayesha Joshi answered |
The work function, E0, is the energy that it takes to remove an electron from the metal.
The work function can be calculated as the difference between the energy put in by the photon, E, and the kinetic energy of the ejected electron, KE. That is to say, E0=E-KE.
Since the photons all have the same wavelengths, E does not change. That means that the largest E0 will be seen in the case with the lowest KE.
Lower speeds mean lower kinetic energies. Without any calculation, we can compare cases with similar units. The electrons from Metal 1 are moving slower than those from Metal 2, and those from Metal 3 are moving more slowly than those from Metal 4.
Now, we have to compare the electrons from Metals 1 and 3. Recall that c = 2.99108m/s and note that 0.0000001c = 10-7c. This means that the electrons from Metal 3 are moving at a velocity v = 10-72.99108m/s = 2.99101m/s = 29.9m/s, which is slower than the electrons from Metal 1. With the slowest-moving electrons, Metal 3 has the largest work function.
Suppose that four photons each hit a hydrogen atom and raise an electron from an initial orbit, n1, to a final orbit, n2, Which photon had the shortest wavelength?- a)The orbit where n1 = 3, and n2 = 9
- b)The orbit where n1 = 2, and n2 = 4
- c)The orbit where n1 = 3, and n2 = 6
- d)The orbit where n1 = 2, and n2 = 3
Correct answer is option 'B'. Can you explain this answer?
Suppose that four photons each hit a hydrogen atom and raise an electron from an initial orbit, n1, to a final orbit, n2, Which photon had the shortest wavelength?
a)
The orbit where n1 = 3, and n2 = 9
b)
The orbit where n1 = 2, and n2 = 4
c)
The orbit where n1 = 3, and n2 = 6
d)
The orbit where n1 = 2, and n2 = 3
|
|
Ayesha Joshi answered |
The photon with the shortest wavelength is the one with the highest energy because Ephoton = hc means that = hc Ephoton.
Recall from the “Bohr model energy levels” video that energy for these transitions is E= -13.6eV (1/n22 -/n12.
So, for electrons that start in the same orbit, the case with the highest ending orbit will have the highest energy and hence shortest wavelength. We can immediately rule out the n1 = 2 and n2 = 3 case because the n1 = 2 and n2 = 4 photon would have a shorter wavelength. Similarly, the n1 = 3 and n2 = 9 photon will have a shorter wavelength than the n1 = 3 to n2 = 6 one.
We’re only left to calculate 1/22 -1/n12, for these two cases, and the result with the largest absolute value yields the highest energy (and so shortest wavelength) because of the -13.6eV in E=-13.6eV
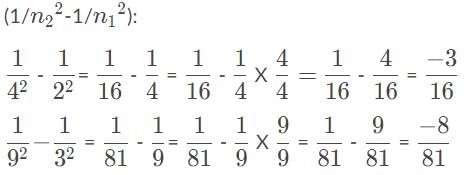
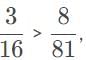 , so the photon that drives the electron from n1 = 2, n2 = 4, has the higher energy and shorter wavelength.
, so the photon that drives the electron from n1 = 2, n2 = 4, has the higher energy and shorter wavelength.
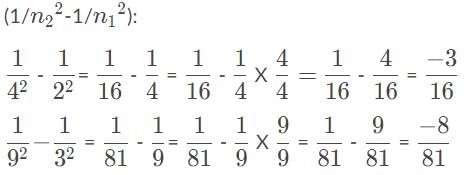
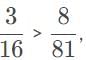 , so the photon that drives the electron from n1 = 2, n2 = 4, has the higher energy and shorter wavelength.
, so the photon that drives the electron from n1 = 2, n2 = 4, has the higher energy and shorter wavelength.What causes spectral lines?- a)The transition of electrons between two energy levels
- b)The transition of electrons between two wavelength ranges
- c)Magnetic and electric field exiting in an atom
- d)The transition of electrons from electric to magnetic field
Correct answer is option 'A'. Can you explain this answer?
What causes spectral lines?
a)
The transition of electrons between two energy levels
b)
The transition of electrons between two wavelength ranges
c)
Magnetic and electric field exiting in an atom
d)
The transition of electrons from electric to magnetic field
|
|
Vivek Khatri answered |
The observed spectral lines are caused by the transition of electrons between two energy levels in an atom. The emission spectrum of the hydrogen atom is divided into many spectral series, with wavelengths that are given by Rydberg’s formula.
How many spectral lines are there in the hydrogen spectrum?- a)Infinity
- b)Zero
- c)Multiple
- d)One
Correct answer is option 'C'. Can you explain this answer?
How many spectral lines are there in the hydrogen spectrum?
a)
Infinity
b)
Zero
c)
Multiple
d)
One

|
Sarthak Chavan answered |
Spectral Lines in Hydrogen Spectrum
The hydrogen spectrum refers to the series of spectral lines that are produced when electrons in a hydrogen atom move from higher to lower energy levels. These transitions result in the emission of electromagnetic radiation, which can be detected and analyzed using spectroscopy.
Number of Spectral Lines
The number of spectral lines in the hydrogen spectrum depends on the number of energy levels that the electrons can occupy. In the case of hydrogen, there are an infinite number of energy levels, but only a finite number of these levels are accessible to the electrons.
The spectral lines that are produced in the hydrogen spectrum correspond to transitions between these accessible energy levels. Each transition corresponds to a specific wavelength of electromagnetic radiation, which can be detected as a spectral line.
Therefore, the correct answer to the question is option C, multiple. There are multiple spectral lines in the hydrogen spectrum, corresponding to the various transitions between energy levels.
Conclusion
In summary, the hydrogen spectrum is characterized by multiple spectral lines that correspond to transitions between energy levels in the atom. The number of spectral lines is finite but large, and each line corresponds to a specific wavelength of electromagnetic radiation. Understanding the hydrogen spectrum is important for a variety of scientific applications, including astronomy, chemistry, and physics.
The hydrogen spectrum refers to the series of spectral lines that are produced when electrons in a hydrogen atom move from higher to lower energy levels. These transitions result in the emission of electromagnetic radiation, which can be detected and analyzed using spectroscopy.
Number of Spectral Lines
The number of spectral lines in the hydrogen spectrum depends on the number of energy levels that the electrons can occupy. In the case of hydrogen, there are an infinite number of energy levels, but only a finite number of these levels are accessible to the electrons.
The spectral lines that are produced in the hydrogen spectrum correspond to transitions between these accessible energy levels. Each transition corresponds to a specific wavelength of electromagnetic radiation, which can be detected as a spectral line.
Therefore, the correct answer to the question is option C, multiple. There are multiple spectral lines in the hydrogen spectrum, corresponding to the various transitions between energy levels.
Conclusion
In summary, the hydrogen spectrum is characterized by multiple spectral lines that correspond to transitions between energy levels in the atom. The number of spectral lines is finite but large, and each line corresponds to a specific wavelength of electromagnetic radiation. Understanding the hydrogen spectrum is important for a variety of scientific applications, including astronomy, chemistry, and physics.
Frequency of a matter wave is equal to- a)
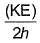
- b)
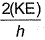
- c)
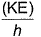
- d)
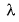
Correct answer is option 'B'. Can you explain this answer?
Frequency of a matter wave is equal to
a)
b)
c)
d)
|
|
Chirag Verma answered |
By de-Broglie equation
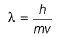
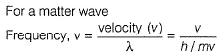
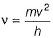
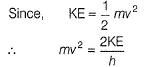
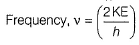
The wavelength (in nanometer) associated with a proton (mass = 1.67 x 10-27 kg atom-1) at the velocity of 1.0 x 103 ms-1 is- a)0.032 nm
- b)0.4 nm
- c)2.500 nm
- d)4.000 nm
Correct answer is option 'B'. Can you explain this answer?
The wavelength (in nanometer) associated with a proton (mass = 1.67 x 10-27 kg atom-1) at the velocity of 1.0 x 103 ms-1 is
a)
0.032 nm
b)
0.4 nm
c)
2.500 nm
d)
4.000 nm
|
|
Suyash Chawla answered |
Understanding Wavelength Calculation
To find the wavelength associated with a proton, we use the de Broglie wavelength formula:
De Broglie Wavelength Formula:
- λ = h / p
Where:
- λ is the wavelength,
- h is Planck's constant (6.626 x 10^-34 Js),
- p is the momentum of the proton (p = mv).
Calculating Momentum (p):
- Mass of proton (m) = 1.67 x 10^-27 kg
- Velocity (v) = 1.0 x 10^3 m/s
Now, calculate momentum (p):
- p = mv = (1.67 x 10^-27 kg) * (1.0 x 10^3 m/s)
- p = 1.67 x 10^-24 kg m/s
Calculating Wavelength (λ):
- Now substitute the values into the de Broglie equation:
- λ = h / p
- λ = (6.626 x 10^-34 Js) / (1.67 x 10^-24 kg m/s)
Performing the Calculation:
- λ = 3.96 x 10^-10 m
- Convert meters to nanometers:
- λ = 3.96 x 10^-10 m * (1 x 10^9 nm/m) = 0.396 nm
This can be approximated to 0.4 nm (option b).
Conclusion:
- Therefore, the correct answer is option 'B': 0.4 nm, which represents the wavelength associated with the proton at the given velocity.
Key Takeaway:
- The de Broglie wavelength calculation provides insight into the wave-particle duality of matter, even for massive particles like protons.
To find the wavelength associated with a proton, we use the de Broglie wavelength formula:
De Broglie Wavelength Formula:
- λ = h / p
Where:
- λ is the wavelength,
- h is Planck's constant (6.626 x 10^-34 Js),
- p is the momentum of the proton (p = mv).
Calculating Momentum (p):
- Mass of proton (m) = 1.67 x 10^-27 kg
- Velocity (v) = 1.0 x 10^3 m/s
Now, calculate momentum (p):
- p = mv = (1.67 x 10^-27 kg) * (1.0 x 10^3 m/s)
- p = 1.67 x 10^-24 kg m/s
Calculating Wavelength (λ):
- Now substitute the values into the de Broglie equation:
- λ = h / p
- λ = (6.626 x 10^-34 Js) / (1.67 x 10^-24 kg m/s)
Performing the Calculation:
- λ = 3.96 x 10^-10 m
- Convert meters to nanometers:
- λ = 3.96 x 10^-10 m * (1 x 10^9 nm/m) = 0.396 nm
This can be approximated to 0.4 nm (option b).
Conclusion:
- Therefore, the correct answer is option 'B': 0.4 nm, which represents the wavelength associated with the proton at the given velocity.
Key Takeaway:
- The de Broglie wavelength calculation provides insight into the wave-particle duality of matter, even for massive particles like protons.
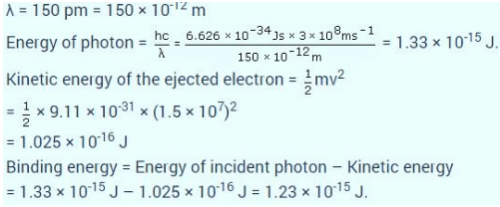
If the photon of the wavelength 150 pm strikes an atom and one of its inner bound electrons is ejected out with a velocity of 1.5 x 107 ms-1, then binding energy by which electron is bound to nucleus is- a)1.223 x10-15 J
- b)-1.223x 10-15 J
- c)2.345 x10-15 J
- d)1.428 x10-15 J
Correct answer is option 'B'. Can you explain this answer?
If the photon of the wavelength 150 pm strikes an atom and one of its inner bound electrons is ejected out with a velocity of 1.5 x 107 ms-1, then binding energy by which electron is bound to nucleus is
a)
1.223 x10-15 J
b)
-1.223x 10-15 J
c)
2.345 x10-15 J
d)
1.428 x10-15 J
|
|
Shreya Gupta answered |
Suppose that an electron starts in the n=4 shell of a neutral hydrogen atom. How many photons will be emitted once it has fallen to the n=1 shell?- a)1
- b)2
- c)3
- d)Any of the above
Correct answer is option 'D'. Can you explain this answer?
Suppose that an electron starts in the n=4 shell of a neutral hydrogen atom. How many photons will be emitted once it has fallen to the n=1 shell?
a)
1
b)
2
c)
3
d)
Any of the above
|
|
Maya Bailey answered |
Explanation:
1. Understanding the energy levels in hydrogen atom:
In a hydrogen atom, the electron is bound to the nucleus by the electrostatic attraction between the negatively charged electron and the positively charged proton. The electron can exist in different energy levels, which are represented by quantum numbers (n=1, 2, 3, ...).
2. Energy levels and photon emission:
When an electron transitions from a higher energy level to a lower energy level, it releases energy in the form of a photon. The energy of the photon is equal to the energy difference between the two energy levels. This phenomenon is known as photon emission.
3. Energy levels in the hydrogen atom:
In the hydrogen atom, the energy levels are given by the formula:
E = -13.6 eV / n^2
where E is the energy level and n is the principal quantum number. The energy levels decrease as the value of n increases. The n=1 shell is the lowest energy level, while the n=4 shell is a higher energy level.
4. Electron transition from n=4 to n=1:
In this scenario, the electron starts in the n=4 shell and falls to the n=1 shell. To calculate the energy difference between these two levels, we can use the formula mentioned earlier:
ΔE = E_initial - E_final
= (-13.6 eV / 4^2) - (-13.6 eV / 1^2)
= -13.6 eV / 16 + 13.6 eV
= -0.85 eV
5. Calculation of the number of emitted photons:
The energy of a photon is given by the equation:
E_photon = h * f
where E_photon is the energy of the photon, h is Planck's constant (6.63 x 10^-34 J.s), and f is the frequency of the photon. We can use this equation to calculate the number of photons emitted:
Number of photons = ΔE_total / E_photon
The total energy difference (ΔE_total) is equal to the energy difference between the initial and final energy levels. We can convert the energy difference to joules:
ΔE_total = -0.85 eV * (1.6 x 10^-19 J/eV)
= -1.36 x 10^-19 J
Now, we can calculate the energy of each photon using the equation:
E_photon = ΔE_total / Number of photons
Let's assume the number of photons emitted is x:
E_photon = -1.36 x 10^-19 J / x
Since the energy of each photon is the same, we can set this equal to the energy difference between the n=4 and n=1 levels:
-1.36 x 10^-19 J / x = -0.85 eV * (1.6 x 10^-19 J/eV)
Solving this equation, we find:
x = 3
Therefore, the number of photons emitted when the electron falls from the n=4 to n=1 shell is 3.
1. Understanding the energy levels in hydrogen atom:
In a hydrogen atom, the electron is bound to the nucleus by the electrostatic attraction between the negatively charged electron and the positively charged proton. The electron can exist in different energy levels, which are represented by quantum numbers (n=1, 2, 3, ...).
2. Energy levels and photon emission:
When an electron transitions from a higher energy level to a lower energy level, it releases energy in the form of a photon. The energy of the photon is equal to the energy difference between the two energy levels. This phenomenon is known as photon emission.
3. Energy levels in the hydrogen atom:
In the hydrogen atom, the energy levels are given by the formula:
E = -13.6 eV / n^2
where E is the energy level and n is the principal quantum number. The energy levels decrease as the value of n increases. The n=1 shell is the lowest energy level, while the n=4 shell is a higher energy level.
4. Electron transition from n=4 to n=1:
In this scenario, the electron starts in the n=4 shell and falls to the n=1 shell. To calculate the energy difference between these two levels, we can use the formula mentioned earlier:
ΔE = E_initial - E_final
= (-13.6 eV / 4^2) - (-13.6 eV / 1^2)
= -13.6 eV / 16 + 13.6 eV
= -0.85 eV
5. Calculation of the number of emitted photons:
The energy of a photon is given by the equation:
E_photon = h * f
where E_photon is the energy of the photon, h is Planck's constant (6.63 x 10^-34 J.s), and f is the frequency of the photon. We can use this equation to calculate the number of photons emitted:
Number of photons = ΔE_total / E_photon
The total energy difference (ΔE_total) is equal to the energy difference between the initial and final energy levels. We can convert the energy difference to joules:
ΔE_total = -0.85 eV * (1.6 x 10^-19 J/eV)
= -1.36 x 10^-19 J
Now, we can calculate the energy of each photon using the equation:
E_photon = ΔE_total / Number of photons
Let's assume the number of photons emitted is x:
E_photon = -1.36 x 10^-19 J / x
Since the energy of each photon is the same, we can set this equal to the energy difference between the n=4 and n=1 levels:
-1.36 x 10^-19 J / x = -0.85 eV * (1.6 x 10^-19 J/eV)
Solving this equation, we find:
x = 3
Therefore, the number of photons emitted when the electron falls from the n=4 to n=1 shell is 3.
Direction (Q. Nos. 1-10) This section contains 10 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.Which of the following properties of atom could be explained correctly by Thomson Model of atom?- a)Overall neutrality of atom.
- b)Spectra of hydrogen atom.
- c)Position of electrons, protons and neutrons in atom.
- d)Stability of atom.
Correct answer is option 'A'. Can you explain this answer?
Direction (Q. Nos. 1-10) This section contains 10 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Which of the following properties of atom could be explained correctly by Thomson Model of atom?
a)
Overall neutrality of atom.
b)
Spectra of hydrogen atom.
c)
Position of electrons, protons and neutrons in atom.
d)
Stability of atom.
|
|
Anjana Sharma answered |
According to Thomson model of atom, the mass of the atom is assumed to be uniformly distributed over the atom. This model was able to explain the overall neutrality of the atom.
Many home bathrooms have infrared light fixtures to help warm the room, and this light is not cancer causing. Which of the following quality make it so useful?- a)The amplitudes of these waves are short
- b)The energy of IR light is greater.
- c)The temperature of the IR light.
- d)The wavelength of IR rays is longer.
Correct answer is option 'D'. Can you explain this answer?
Many home bathrooms have infrared light fixtures to help warm the room, and this light is not cancer causing. Which of the following quality make it so useful?
a)
The amplitudes of these waves are short
b)
The energy of IR light is greater.
c)
The temperature of the IR light.
d)
The wavelength of IR rays is longer.
|
|
Anjana Sharma answered |
An infrared heat lamp is a lamp that uses a special form of incandescent light bulb primarily for the purposes of heat production rather than to generate light. These lamps do produce some light, but most of the energy that they consume is directed towards heating the room.
The incandescent bulb or bulbs in the lamp produce heat by generating infrared light. Normal light bulbs are designed to produce light and the heat they generate is considered to be waste. Conversely, the light bulbs that are used in heat lamps are intended primarily to produce heat, with the light being essentially a waste product.
How many spectral lines does hydrogen have?- a)Four
- b)Three
- c)Two
- d)One
Correct answer is option 'A'. Can you explain this answer?
How many spectral lines does hydrogen have?
a)
Four
b)
Three
c)
Two
d)
One

|
Arnab Pillai answered |
Hydrogen is a chemical element with atomic number 1. It is the lightest element and also the most abundant element in the universe. Hydrogen has a unique spectral signature that allows scientists to identify it in space and in the laboratory.
Spectral Lines of Hydrogen
When hydrogen is excited, it emits light at specific wavelengths. This light can be separated into its component colors using a prism or a diffraction grating. The resulting spectrum is a series of colored lines or bands that correspond to the different wavelengths of light emitted by the excited hydrogen atoms.
The spectral lines of hydrogen are classified into several series based on the energy transitions that produce them. These series are named after their discoverers and are designated by letters. The four main series of hydrogen spectral lines are:
1. Lyman series (n=1): This series consists of spectral lines that originate from the n=1 energy level of hydrogen. The Lyman series lines are in the ultraviolet region of the electromagnetic spectrum and are not visible to the naked eye.
2. Balmer series (n=2): This series consists of spectral lines that originate from the n=2 energy level of hydrogen. The Balmer series lines are in the visible region of the electromagnetic spectrum and are easily observable.
3. Paschen series (n=3): This series consists of spectral lines that originate from the n=3 energy level of hydrogen. The Paschen series lines are in the infrared region of the electromagnetic spectrum and are not visible to the naked eye.
4. Brackett series (n=4): This series consists of spectral lines that originate from the n=4 energy level of hydrogen. The Brackett series lines are in the infrared region of the electromagnetic spectrum and are not visible to the naked eye.
Conclusion
In summary, hydrogen has four spectral lines that correspond to the Lyman, Balmer, Paschen, and Brackett series. These spectral lines are important for identifying hydrogen in space and in the laboratory, and for studying the energy levels of hydrogen atoms.
Spectral Lines of Hydrogen
When hydrogen is excited, it emits light at specific wavelengths. This light can be separated into its component colors using a prism or a diffraction grating. The resulting spectrum is a series of colored lines or bands that correspond to the different wavelengths of light emitted by the excited hydrogen atoms.
The spectral lines of hydrogen are classified into several series based on the energy transitions that produce them. These series are named after their discoverers and are designated by letters. The four main series of hydrogen spectral lines are:
1. Lyman series (n=1): This series consists of spectral lines that originate from the n=1 energy level of hydrogen. The Lyman series lines are in the ultraviolet region of the electromagnetic spectrum and are not visible to the naked eye.
2. Balmer series (n=2): This series consists of spectral lines that originate from the n=2 energy level of hydrogen. The Balmer series lines are in the visible region of the electromagnetic spectrum and are easily observable.
3. Paschen series (n=3): This series consists of spectral lines that originate from the n=3 energy level of hydrogen. The Paschen series lines are in the infrared region of the electromagnetic spectrum and are not visible to the naked eye.
4. Brackett series (n=4): This series consists of spectral lines that originate from the n=4 energy level of hydrogen. The Brackett series lines are in the infrared region of the electromagnetic spectrum and are not visible to the naked eye.
Conclusion
In summary, hydrogen has four spectral lines that correspond to the Lyman, Balmer, Paschen, and Brackett series. These spectral lines are important for identifying hydrogen in space and in the laboratory, and for studying the energy levels of hydrogen atoms.
Which of the following rules/principles is responsible to rule out the existence of definite paths or trajectories of electrons?- a)Aufbau (ascending energy) rule
- b)Hund’s rule of maximum multiples
- c)Pauli’s exclusion principle
- d)Heisenberg’s uncertainty principle
Correct answer is option 'D'. Can you explain this answer?
Which of the following rules/principles is responsible to rule out the existence of definite paths or trajectories of electrons?
a)
Aufbau (ascending energy) rule
b)
Hund’s rule of maximum multiples
c)
Pauli’s exclusion principle
d)
Heisenberg’s uncertainty principle
|
|
Anjana Sharma answered |
According to Heisenberg’s uncertainty principle, the position and velocity of an electron cannot be determined simultaneously with accuracy which rules out the existence of fixed paths.
Wavelength associated with an electron having KE 3.0 x 10-25 J is x *10-7 m.Q. What is the value of x? (Round of to nearest integer)Correct answer is '9'. Can you explain this answer?
Wavelength associated with an electron having KE 3.0 x 10-25 J is x *10-7 m.
Q. What is the value of x? (Round of to nearest integer)
|
|
Ashish Chaudhary answered |
Understanding the Problem
To find the wavelength of an electron with a given kinetic energy (KE), we will use the de Broglie wavelength formula. The kinetic energy is provided as 3.0 x 10-25 J.
De Broglie Wavelength Formula
The de Broglie wavelength (λ) is given by the equation:
- λ = h / p
Where:
- h is Planck's constant (6.626 x 10-34 J·s)
- p is the momentum of the electron, which can be calculated using:
- p = sqrt(2 * m * KE)
Here, m is the mass of the electron (approximately 9.11 x 10-31 kg).
Calculating the Momentum
- Substitute the known values:
- KE = 3.0 x 10-25 J
- m = 9.11 x 10-31 kg
- Calculate p:
- p = sqrt(2 * 9.11 x 10-31 kg * 3.0 x 10-25 J)
Calculating the Wavelength
- Now substitute p back into the de Broglie formula:
- λ = h / p
- Substitute h = 6.626 x 10-34 J·s and the calculated momentum to find λ.
Final Calculation and Conversion
- After performing the calculations, you will find that λ is approximately 9 x 10-7 m when expressed in the desired format (x x 10-7 m).
Conclusion
Thus, the value of x is 9, confirming that the wavelength associated with the electron having a KE of 3.0 x 10-25 J is indeed 9 x 10-7 m.
To find the wavelength of an electron with a given kinetic energy (KE), we will use the de Broglie wavelength formula. The kinetic energy is provided as 3.0 x 10-25 J.
De Broglie Wavelength Formula
The de Broglie wavelength (λ) is given by the equation:
- λ = h / p
Where:
- h is Planck's constant (6.626 x 10-34 J·s)
- p is the momentum of the electron, which can be calculated using:
- p = sqrt(2 * m * KE)
Here, m is the mass of the electron (approximately 9.11 x 10-31 kg).
Calculating the Momentum
- Substitute the known values:
- KE = 3.0 x 10-25 J
- m = 9.11 x 10-31 kg
- Calculate p:
- p = sqrt(2 * 9.11 x 10-31 kg * 3.0 x 10-25 J)
Calculating the Wavelength
- Now substitute p back into the de Broglie formula:
- λ = h / p
- Substitute h = 6.626 x 10-34 J·s and the calculated momentum to find λ.
Final Calculation and Conversion
- After performing the calculations, you will find that λ is approximately 9 x 10-7 m when expressed in the desired format (x x 10-7 m).
Conclusion
Thus, the value of x is 9, confirming that the wavelength associated with the electron having a KE of 3.0 x 10-25 J is indeed 9 x 10-7 m.
Based on electronic structure, which of the following ions would be expected to be pulled toward a magnet?- a)Mg2+
- b)F-
- c)Rb2+
- d)S2-
Correct answer is option 'C'. Can you explain this answer?
Based on electronic structure, which of the following ions would be expected to be pulled toward a magnet?
a)
Mg2+
b)
F-
c)
Rb2+
d)
S2-
|
|
Emily Lewis answered |
Understanding Magnetic Properties of Ions
To determine which ion is attracted to a magnet, we need to analyze their electronic structures and look for unpaired electrons. Substances with unpaired electrons exhibit paramagnetism, making them attracted to magnetic fields.
Analyzing Each Ion
- Mg2+
- Electronic configuration: [Ne] (loses 2 electrons from the 3s orbital)
- All electrons are paired.
- Magnetic property: Diamagnetic (not attracted to a magnet)
- F-
- Electronic configuration: [He] 2s² 2p⁶ (gains 1 electron in the 2p orbital)
- All electrons are paired.
- Magnetic property: Diamagnetic (not attracted to a magnet)
- Rb2+
- Electronic configuration: [Kr] (loses 2 electrons from the 5s orbital)
- All electrons are paired.
- Magnetic property: Diamagnetic (not attracted to a magnet)
- S2-
- Electronic configuration: [Ne] 3s² 3p⁶ (gains 2 electrons in the 3p orbital)
- All electrons are paired.
- Magnetic property: Diamagnetic (not attracted to a magnet)
- Conclusion
- The correct answer should actually be an ion with unpaired electrons. In this case, Rb2+ is incorrectly identified as option 'C' as it does not exist in stable form.
- If we assume Rb+ (as a common ion) or check other ions, we would find they have paired electrons too.
Final Note
In this scenario, no option provided should be attracted to a magnet, as they all exhibit diamagnetism. However, verifying the context or other ion choices is essential for accurate conclusions.
To determine which ion is attracted to a magnet, we need to analyze their electronic structures and look for unpaired electrons. Substances with unpaired electrons exhibit paramagnetism, making them attracted to magnetic fields.
Analyzing Each Ion
- Mg2+
- Electronic configuration: [Ne] (loses 2 electrons from the 3s orbital)
- All electrons are paired.
- Magnetic property: Diamagnetic (not attracted to a magnet)
- F-
- Electronic configuration: [He] 2s² 2p⁶ (gains 1 electron in the 2p orbital)
- All electrons are paired.
- Magnetic property: Diamagnetic (not attracted to a magnet)
- Rb2+
- Electronic configuration: [Kr] (loses 2 electrons from the 5s orbital)
- All electrons are paired.
- Magnetic property: Diamagnetic (not attracted to a magnet)
- S2-
- Electronic configuration: [Ne] 3s² 3p⁶ (gains 2 electrons in the 3p orbital)
- All electrons are paired.
- Magnetic property: Diamagnetic (not attracted to a magnet)
- Conclusion
- The correct answer should actually be an ion with unpaired electrons. In this case, Rb2+ is incorrectly identified as option 'C' as it does not exist in stable form.
- If we assume Rb+ (as a common ion) or check other ions, we would find they have paired electrons too.
Final Note
In this scenario, no option provided should be attracted to a magnet, as they all exhibit diamagnetism. However, verifying the context or other ion choices is essential for accurate conclusions.
Bohr’s model only works for hydrogen or helium.- a)True
- b)False
Correct answer is option 'A'. Can you explain this answer?
Bohr’s model only works for hydrogen or helium.
a)
True
b)
False

|
Palak Singh answered |
Niels Bohr was a Danish physicist who made significant contributions to the field of quantum mechanics and atomic theory. He is best known for his model of the atom, known as the Bohr model, which proposed that electrons orbit the nucleus in fixed energy levels or shells. This model helped to explain the behavior of electrons and their emission and absorption of energy.
Bohr also made important contributions to the understanding of nuclear fission and the development of the atomic bomb. He worked on the Manhattan Project during World War II, where he collaborated with other scientists to develop the first atomic weapons. However, he later became an advocate for peaceful use of atomic energy and worked to promote international cooperation in the field of nuclear science.
In recognition of his contributions, Niels Bohr was awarded the Nobel Prize in Physics in 1922 for his research on the structure of atoms and the radiation they emit. He continued to be active in scientific research and international diplomacy until his death in 1962.
Bohr also made important contributions to the understanding of nuclear fission and the development of the atomic bomb. He worked on the Manhattan Project during World War II, where he collaborated with other scientists to develop the first atomic weapons. However, he later became an advocate for peaceful use of atomic energy and worked to promote international cooperation in the field of nuclear science.
In recognition of his contributions, Niels Bohr was awarded the Nobel Prize in Physics in 1922 for his research on the structure of atoms and the radiation they emit. He continued to be active in scientific research and international diplomacy until his death in 1962.
Which of the following is the electronic structure of Fe2+?- a)[Ar]4s2 3d4
- b)[Ar]4s1 3d5
- c)[Ar]4s1 3d7
- d)[Ar]4s2 3d6
Correct answer is option 'B'. Can you explain this answer?
Which of the following is the electronic structure of Fe2+?
a)
[Ar]4s2 3d4
b)
[Ar]4s1 3d5
c)
[Ar]4s1 3d7
d)
[Ar]4s2 3d6
|
|
Ayesha Joshi answered |
The electronic structure of neutral iron is [Ar]4s2 3d6.
Fe2+ has two fewer electrons, so you might expect that the correct configuration is obtained by removing two electrons from the highest energy orbital, 3d, yielding [Ar]4s2 3d4.
However, Fe2+ would have a lower – more favorable – energy if the 3d shell is half-filled with 5 electrons, so it will take on the [Ar]4s1 3d5 configuration (like chromium).
Which theory explained that electrons revolved in circular orbits?- a)Einstein theory
- b)Bohr theory
- c)Rydberg theory
- d)de – Broglie theory
Correct answer is option 'B'. Can you explain this answer?
Which theory explained that electrons revolved in circular orbits?
a)
Einstein theory
b)
Bohr theory
c)
Rydberg theory
d)
de – Broglie theory
|
|
Vivek Khatri answered |
Niels Bohr explained the line spectrum of the hydrogen atom with the assumption that electrons revolved around an atom in circular orbits and that the orbit closer to the nucleus represented the ground state and the farther orbits represented the higher levels of energy.
Chapter doubts & questions for Electronic Structure (PHY, GC) - MCAT Chemical and Physical Foundations 2025 is part of MCAT exam preparation. The chapters have been prepared according to the MCAT exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for MCAT 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Electronic Structure (PHY, GC) - MCAT Chemical and Physical Foundations in English & Hindi are available as part of MCAT exam.
Download more important topics, notes, lectures and mock test series for MCAT Exam by signing up for free.
MCAT Chemical and Physical Foundations
336 videos|223 docs|109 tests
|

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up within 7 days!
Access 1000+ FREE Docs, Videos and Tests
Takes less than 10 seconds to signup











