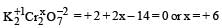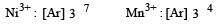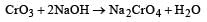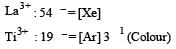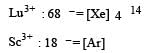All Exams >
NEET >
Chemistry 31 Years NEET Chapterwise Solved Papers >
All Questions
All questions of The d and f-Block Elements for NEET Exam
Photographic films and plates have an essential ingredient of [1989]- a)Silver nitrate
- b)Silver bromide
- c)Sodium chloride
- d)Oleic acid
Correct answer is option 'B'. Can you explain this answer?
Photographic films and plates have an essential ingredient of [1989]
a)
Silver nitrate
b)
Silver bromide
c)
Sodium chloride
d)
Oleic acid

|
Abhiram Nair answered |
AgBr is highly photosensitive and is used in photographic films and plates.
The transition elements have a general electronic configuration [1991]- a)ns2, np6, nd1-10
- b)(n - 1) d1-10 , ns0 - 2 , np0 - 6
- c)(n - 1) d1-10 , ns1- 2
- d)n d1 -10 , ns-2
Correct answer is option 'C'. Can you explain this answer?
The transition elements have a general electronic configuration [1991]
a)
ns2, np6, nd1-10
b)
(n - 1) d1-10 , ns0 - 2 , np0 - 6
c)
(n - 1) d1-10 , ns1- 2
d)
n d1 -10 , ns-2

|
Abhiram Nair answered |
General electronic configuration of transition elements is (n - 1)d1 -10ns1- 2
Which of the following shows maximum number of oxidation states? [2002]- a)Cr
- b)Fe
- c)Mn
- d)V
Correct answer is option 'C'. Can you explain this answer?
Which of the following shows maximum number of oxidation states? [2002]
a)
Cr
b)
Fe
c)
Mn
d)
V

|
Arpita Tiwari answered |
Mn : [Ar] 3d5 4s2
Shows +2, +3, +4, +5, +6 & +7 oxidation states
Shows +2, +3, +4, +5, +6 & +7 oxidation states
For the four successive transition elements (Cr, Mn, Fe and Co), the stability of +2 oxidation state will be there in which of the following order?- a)Mn > Fe > Cr > Co [2011]
- b)Fe > Mn > Co > Cr
- c)Co > Mn > Fe > Cr
- d)Cr > Mn > Co > Fe
Correct answer is option 'A'. Can you explain this answer?
For the four successive transition elements (Cr, Mn, Fe and Co), the stability of +2 oxidation state will be there in which of the following order?
a)
Mn > Fe > Cr > Co [2011]
b)
Fe > Mn > Co > Cr
c)
Co > Mn > Fe > Cr
d)
Cr > Mn > Co > Fe
|
|
Kalyan Chavan answered |
B)Fe
c)Co
d)Cr
The correct answer is: d) Cr
The stability of the +2 oxidation state decreases as we move from Cr to Co. This is because the effective nuclear charge experienced by the valence electrons increases as we move from left to right in a period, making it more difficult for the element to lose electrons and form a positive ion. Additionally, the electron configuration of Cr2+ (3d4) is more stable than that of Mn2+ (3d5), Fe2+ (3d6), and Co2+ (3d7) due to half-filled and fully-filled subshells being more stable. Therefore, the stability of the +2 oxidation state follows the order Cr > Mn > Fe > Co.
c)Co
d)Cr
The correct answer is: d) Cr
The stability of the +2 oxidation state decreases as we move from Cr to Co. This is because the effective nuclear charge experienced by the valence electrons increases as we move from left to right in a period, making it more difficult for the element to lose electrons and form a positive ion. Additionally, the electron configuration of Cr2+ (3d4) is more stable than that of Mn2+ (3d5), Fe2+ (3d6), and Co2+ (3d7) due to half-filled and fully-filled subshells being more stable. Therefore, the stability of the +2 oxidation state follows the order Cr > Mn > Fe > Co.
The addition of excess of aqueous HNO3 to a solution containing [Cu(NH3)4]2+ produces [1999]- a)Cu+
- b)[Cu(H2O)4]2+
- c)Cu(OH)2
- d)Cu(NO3)2
Correct answer is option 'B'. Can you explain this answer?
The addition of excess of aqueous HNO3 to a solution containing [Cu(NH3)4]2+ produces [1999]
a)
Cu+
b)
[Cu(H2O)4]2+
c)
Cu(OH)2
d)
Cu(NO3)2

|
Prisha Singh answered |
[Cu (NH3)4]2+ on addition of excess of aqneous HNO3 gives [Cu (H2O)4]2+
A blue colouration is not obtained when [1989]- a)Ammonium hydroxide dissolves in copper sulphate
- b)Coppersulp hate solution reacts with K4[Fe(CN)6]
- c)Ferric chloride reacts with sod. ferrocyanide
- d)Anhydrous CuSO4 is dissolved in water
Correct answer is option 'B'. Can you explain this answer?
A blue colouration is not obtained when [1989]
a)
Ammonium hydroxide dissolves in copper sulphate
b)
Coppersulp hate solution reacts with K4[Fe(CN)6]
c)
Ferric chloride reacts with sod. ferrocyanide
d)
Anhydrous CuSO4 is dissolved in water

|
Lekshmi Banerjee answered |
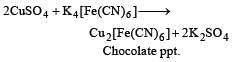
Among the lanthanides the one obtained by synthetic method is [1994]- a)Lu
- b)Pm
- c)Pr
- d)Gd
Correct answer is option 'B'. Can you explain this answer?
Among the lanthanides the one obtained by synthetic method is [1994]
a)
Lu
b)
Pm
c)
Pr
d)
Gd

|
Jatin Chakraborty answered |
Pm is obtained by synthetic method.
The catalytic activity of transition metals and their compounds is ascribed mainly to :[2012 M]- a)their magnetic behaviour
- b)their unfilled d-orbitals
- c)their ability to adopt variable oxidation state
- d)their chemical reactivity
Correct answer is option 'C'. Can you explain this answer?
The catalytic activity of transition metals and their compounds is ascribed mainly to :[2012 M]
a)
their magnetic behaviour
b)
their unfilled d-orbitals
c)
their ability to adopt variable oxidation state
d)
their chemical reactivity

|
Maheshwar Saini answered |
The transition metals and their compounds are used as catalysts. Because of the variable oxidation states, due to this, they easily absorb and re-emit wide range of energy to provide the necessary activation energy.
Stainless steel contains iron and [1995]- a)Cr + Ni
- b)Cr + Zn
- c)Zn + Pb
- d)Fe +Cr + Ni
Correct answer is option 'D'. Can you explain this answer?
Stainless steel contains iron and [1995]
a)
Cr + Ni
b)
Cr + Zn
c)
Zn + Pb
d)
Fe +Cr + Ni

|
Sonal Kulkarni answered |
Stainless steel contains 73% Fe, 18% Cr and 8% Ni.
The correctorder of decreasing second ionisation enthalpy of Ti (22), V(23), Cr(24) and Mn (25) is : [2008]- a)Cr > Mn > V > Ti
- b)V > Mn > Cr > Ti
- c)Mn > Cr > Ti > V
- d)Ti > V > Cr > Mn
Correct answer is option 'A'. Can you explain this answer?
The correctorder of decreasing second ionisation enthalpy of Ti (22), V(23), Cr(24) and Mn (25) is : [2008]
a)
Cr > Mn > V > Ti
b)
V > Mn > Cr > Ti
c)
Mn > Cr > Ti > V
d)
Ti > V > Cr > Mn

|
Nilotpal Gupta answered |
Ti ; Z (22) is 1s22s22p63s23p64s23d2
V ; Z (23) is 1s22s22p63s23p64s23d3
Cr ; Z (24) is 1s22s22p63s23p63d54s1
Mn ; Z (25) is 1s22s22p63s23d54s2
V ; Z (23) is 1s22s22p63s23p64s23d3
Cr ; Z (24) is 1s22s22p63s23p63d54s1
Mn ; Z (25) is 1s22s22p63s23d54s2
The second electron in all the cases (except Cr) is taken out from 4s-orbital and for Cr it is an electron from completely half filled 3d-orbital. The force required for removal of second electron will be more for Mn (except for Cr) having more positive charge.
Based on this we find the correct order is Mn > V > Ti. Cr > Mn > V > Ti. So correct answeris (a).
Based on this we find the correct order is Mn > V > Ti. Cr > Mn > V > Ti. So correct answeris (a).
In which of the following pairs are both the ions coloured in aqueous solutions ? [2006] (At. no. : Sc = 21, Ti = 22, Ni = 28, Cu = 29, Co = 27)- a)Sc3+, Ti3+
- b)Sc3+, Co2+
- c)Ni2+, Cu+
- d)Ni2+, Ti3+
Correct answer is option 'D'. Can you explain this answer?
In which of the following pairs are both the ions coloured in aqueous solutions ? [2006] (At. no. : Sc = 21, Ti = 22, Ni = 28, Cu = 29, Co = 27)
a)
Sc3+, Ti3+
b)
Sc3+, Co2+
c)
Ni2+, Cu+
d)
Ni2+, Ti3+

|
Dipanjan Mehta answered |
Sc3+ : 1s2, 2s2p6, 3s2p6d0, 4s0; no unpaired electron.
Cu+ : 1s2, 2s2p6, 3s2p6d10, 4s0; no unpaired electron.
Ni2+: 1s2, 2s2p6, 3s2p6d8, 4s0; unpaired electron present.
Ti3+ : 1s2, 2s2p6, 2s2p6d1, 4s0; unpaired electron present Co2+ : 1s2, 2s2p6, 3s2p6d7, 4s0; unpaired electron present So from the given option the only correct combination is Ni2+ and Ti3+
Cu+ : 1s2, 2s2p6, 3s2p6d10, 4s0; no unpaired electron.
Ni2+: 1s2, 2s2p6, 3s2p6d8, 4s0; unpaired electron present.
Ti3+ : 1s2, 2s2p6, 2s2p6d1, 4s0; unpaired electron present Co2+ : 1s2, 2s2p6, 3s2p6d7, 4s0; unpaired electron present So from the given option the only correct combination is Ni2+ and Ti3+
Lanthanoids are [2004]- a)14 elements in the sixth period (atomic no. = 90 to 103) that are filling 4f sublevel
- b)14 elements in the seventh period (atomic no. = 90 to 103) that are filling 5f sublevel
- c)14 elements in the sixth period (atomic no. = 58 to 71) that are filling 4f sublevel
- d)14 elements in the seventh period (atomic no. = 58 to 71) that are filling 4f sublevel
Correct answer is option 'C'. Can you explain this answer?
Lanthanoids are [2004]
a)
14 elements in the sixth period (atomic no. = 90 to 103) that are filling 4f sublevel
b)
14 elements in the seventh period (atomic no. = 90 to 103) that are filling 5f sublevel
c)
14 elements in the sixth period (atomic no. = 58 to 71) that are filling 4f sublevel
d)
14 elements in the seventh period (atomic no. = 58 to 71) that are filling 4f sublevel

|
Athira Datta answered |
The correct answer is option C: 14 elements in the sixth period (atomic no. = 58 to 71) that are filling the 4f sublevel.
Explanation:
- The periodic table is organized into periods and groups. The periods are horizontal rows, and the groups are vertical columns.
- The lanthanoids, also known as the lanthanides, are a group of elements that belong to the f-block of the periodic table.
- The lanthanoid series consists of 14 elements that fill the 4f sublevel. These elements have atomic numbers ranging from 58 to 71.
- The 4f sublevel is part of the fourth energy level (n=4) and is characterized by its unique electron configuration.
- In the lanthanoid series, the 4f sublevel is gradually filled up as you move across the period. Each element in the series adds one more electron to the 4f sublevel until it is completely filled.
- The lanthanoids are located in the sixth period of the periodic table because they correspond to the filling of the 4f sublevel.
- The lanthanoids are known for their similar chemical properties, which arise from the filling of the 4f sublevel. They are generally soft, malleable metals with high melting points and good electrical conductivity.
- Some common examples of lanthanoids include cerium (Ce), praseodymium (Pr), and europium (Eu).
- It's important to note that the atomic numbers of the lanthanoids range from 58 to 71, not from 90 to 103 as mentioned in options A and B. The atomic numbers from 90 to 103 correspond to a different series of elements called the actinoids, which fill the 5f sublevel.
- Therefore, the correct answer is option C: 14 elements in the sixth period (atomic no. = 58 to 71) that are filling the 4f sublevel.
Explanation:
- The periodic table is organized into periods and groups. The periods are horizontal rows, and the groups are vertical columns.
- The lanthanoids, also known as the lanthanides, are a group of elements that belong to the f-block of the periodic table.
- The lanthanoid series consists of 14 elements that fill the 4f sublevel. These elements have atomic numbers ranging from 58 to 71.
- The 4f sublevel is part of the fourth energy level (n=4) and is characterized by its unique electron configuration.
- In the lanthanoid series, the 4f sublevel is gradually filled up as you move across the period. Each element in the series adds one more electron to the 4f sublevel until it is completely filled.
- The lanthanoids are located in the sixth period of the periodic table because they correspond to the filling of the 4f sublevel.
- The lanthanoids are known for their similar chemical properties, which arise from the filling of the 4f sublevel. They are generally soft, malleable metals with high melting points and good electrical conductivity.
- Some common examples of lanthanoids include cerium (Ce), praseodymium (Pr), and europium (Eu).
- It's important to note that the atomic numbers of the lanthanoids range from 58 to 71, not from 90 to 103 as mentioned in options A and B. The atomic numbers from 90 to 103 correspond to a different series of elements called the actinoids, which fill the 5f sublevel.
- Therefore, the correct answer is option C: 14 elements in the sixth period (atomic no. = 58 to 71) that are filling the 4f sublevel.
Four successive members of the first row transition elements are listed below with their atomic numbers. Which one of them is expected to have the highest third ionization enthalpy? [2 00 5]- a)Vanadium (Z = 23)
- b)Chromium (Z = 24)
- c)Man gan ese (Z = 25)
- d)Ir on (Z = 26)
Correct answer is option 'C'. Can you explain this answer?
Four successive members of the first row transition elements are listed below with their atomic numbers. Which one of them is expected to have the highest third ionization enthalpy? [2 00 5]
a)
Vanadium (Z = 23)
b)
Chromium (Z = 24)
c)
Man gan ese (Z = 25)
d)
Ir on (Z = 26)

|
Ashwini Khanna answered |
For third ion ization enthalpy last configuration of
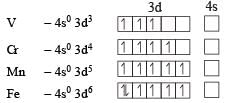
For third Ionization enthalpy Mn has stable configuration due to half filled d-orbital.
Of the following transition metals, the maximum numbers of oxidation states are exhibited by: [2000]- a)Chromiun (Z = 24)
- b)Manganese (Z = 25)
- c)Iron (Z = 26)
- d)Titanium (Z=22)
Correct answer is option 'B'. Can you explain this answer?
Of the following transition metals, the maximum numbers of oxidation states are exhibited by: [2000]
a)
Chromiun (Z = 24)
b)
Manganese (Z = 25)
c)
Iron (Z = 26)
d)
Titanium (Z=22)

|
Shivani Rane answered |
Manganese shows max. no. of oxidation states, +2, +3, + 4, + 5, + 6, + 7
Other metals shows the following oxidation states
Cr = + 2, +3, + 4, + 5, + 6
Fe = + 2, + 3
Ti = + 2, + 3, + 4
Other metals shows the following oxidation states
Cr = + 2, +3, + 4, + 5, + 6
Fe = + 2, + 3
Ti = + 2, + 3, + 4
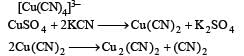
Copper sulphate dissolves in excess of KCN to give[2006]- a)[Cu(CN)4]3–
- b)[Cu(CN)4]2–
- c)Cu(CN)2
- d)CuCN
Correct answer is option 'A'. Can you explain this answer?
Copper sulphate dissolves in excess of KCN to give[2006]
a)
[Cu(CN)4]3–
b)
[Cu(CN)4]2–
c)
Cu(CN)2
d)
CuCN

|
Rajeev Sharma answered |
Copper sulphate on treatment with excess of KCN forms complex K3[Cu(CN)4] or

Which of the following forms colourless compound? [2000]- a)Sc3+
- b)V3+
- c)Ti3+
- d)Cr3+
Correct answer is option 'A'. Can you explain this answer?
Which of the following forms colourless compound? [2000]
a)
Sc3+
b)
V3+
c)
Ti3+
d)
Cr3+
|
|
Nabanita Sen answered |
Sc3+ forms a colourless compound.
- Explanation:
- Scandium (Sc) is a transition metal that typically forms colourless compounds.
- When Scandium loses three electrons to form Sc3+ ion, it does not have any d-electrons to exhibit colour due to the absence of d-d transitions.
- The Sc3+ ion has a completely filled d-orbital, resulting in the absence of any visible absorption of light, making the compound colourless.
- On the other hand, ions like V3+, Ti3+, and Cr3+ have partially filled d-orbitals, which can lead to the absorption of certain wavelengths of light, resulting in coloured compounds.
Therefore, among the given options, Sc3+ is the only one that forms a colourless compound.
- Explanation:
- Scandium (Sc) is a transition metal that typically forms colourless compounds.
- When Scandium loses three electrons to form Sc3+ ion, it does not have any d-electrons to exhibit colour due to the absence of d-d transitions.
- The Sc3+ ion has a completely filled d-orbital, resulting in the absence of any visible absorption of light, making the compound colourless.
- On the other hand, ions like V3+, Ti3+, and Cr3+ have partially filled d-orbitals, which can lead to the absorption of certain wavelengths of light, resulting in coloured compounds.
Therefore, among the given options, Sc3+ is the only one that forms a colourless compound.
Which of the following pairs has the same size?a)Fe2+ , Ni 2+b)Zr 4+ , Ti4+ c)Zr 4+ , Hf 4+d)Zn 2+ , Hf 4+Correct answer is option 'C'. Can you explain this answer?

|
Prasenjit Pillai answered |
Due to lanthanide contraction, the size of Zr and Hf (atom and ions) become nearly similar
The composition of ‘golden spangles’ is [1990]- a)PbCrO4
- b)PbI2
- c)As 2S3
- d)BaCrO4
Correct answer is option 'B'. Can you explain this answer?
The composition of ‘golden spangles’ is [1990]
a)
PbCrO4
b)
PbI2
c)
As 2S3
d)
BaCrO4

|
Nabanita Singh answered |
A substance refers to the types and amounts of different elements or compounds that make up the substance. It describes the chemical makeup of the substance and is often represented using a chemical formula or a percentage composition.
For example, the composition of water (H2O) indicates that it is made up of two hydrogen atoms bonded to one oxygen atom. The composition of carbon dioxide (CO2) indicates that it is made up of one carbon atom bonded to two oxygen atoms.
The composition of a substance can also be expressed in terms of percentage composition, which shows the relative amounts of each element or compound present. For example, the percentage composition of water is 11.1% hydrogen and 88.9% oxygen.
Knowing the composition of a substance is important in various fields, such as chemistry, materials science, and biology, as it helps to understand the properties and behavior of the substance. It can also be used to determine the stoichiometry of chemical reactions and to predict the products of chemical reactions.
For example, the composition of water (H2O) indicates that it is made up of two hydrogen atoms bonded to one oxygen atom. The composition of carbon dioxide (CO2) indicates that it is made up of one carbon atom bonded to two oxygen atoms.
The composition of a substance can also be expressed in terms of percentage composition, which shows the relative amounts of each element or compound present. For example, the percentage composition of water is 11.1% hydrogen and 88.9% oxygen.
Knowing the composition of a substance is important in various fields, such as chemistry, materials science, and biology, as it helps to understand the properties and behavior of the substance. It can also be used to determine the stoichiometry of chemical reactions and to predict the products of chemical reactions.
The aqueous solution containing which one of the following ions will be colourless? (Atomic number: Sc = 21, Fe = 26, Ti = 22, Mn = 25)[2005]- a)Sc3+
- b)Fe2+
- c)Ti3+
- d)Mn 2+
Correct answer is option 'A'. Can you explain this answer?
The aqueous solution containing which one of the following ions will be colourless? (Atomic number: Sc = 21, Fe = 26, Ti = 22, Mn = 25)[2005]
a)
Sc3+
b)
Fe2+
c)
Ti3+
d)
Mn 2+

|
Maheshwar Saini answered |
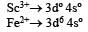

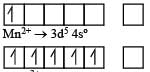
In Sc3+ there as no unpaired electron So the aqueous solution of Sc3+ will be colourless.
The main reason for larger number of oxidation states exhibited by the actinoids than the corresponding lanthanoids, is [2005, 2006]- a)more energy difference between 5f and 6d orbitals than between 4f and 5d orbitals.
- b)lesser energy difference between 5f and 6d orbitals than between 4f and 5d orbitals.
- c)larger atomic size of actinoids than the lanthanoids.
- d)greater reactive nature of the actinoids than the lanthanoids.
Correct answer is option 'B'. Can you explain this answer?
The main reason for larger number of oxidation states exhibited by the actinoids than the corresponding lanthanoids, is [2005, 2006]
a)
more energy difference between 5f and 6d orbitals than between 4f and 5d orbitals.
b)
lesser energy difference between 5f and 6d orbitals than between 4f and 5d orbitals.
c)
larger atomic size of actinoids than the lanthanoids.
d)
greater reactive nature of the actinoids than the lanthanoids.

|
Nilotpal Gupta answered |
The cause of showing different oxidation is due to the fact that there is only a small difference between the energies of electron in the ns orbitals and (n – 1)d orbitals with the result both ns as well (n – 1)d electrons can be used for compound formation. Lesser energy difference between 5f and 6d orbitals than between 4f and 5d orbitals result in larger no. of oxidation state.
Which one of the following elements shows maximum number of different oxidation states in its compounds? [1998]- a)Eu
- b)La
- c)Ge
- d)Am
Correct answer is option 'D'. Can you explain this answer?
Which one of the following elements shows maximum number of different oxidation states in its compounds? [1998]
a)
Eu
b)
La
c)
Ge
d)
Am

|
Deepak Joshi answered |
We know that lanthanides Eu, La, Gd shows +2, +3, +3 and +3 oxidation states respectively only. while actinides, Am shows +2, +3, +4, +5 and +6 oxidation state. Therefore American (Am) has maximum number of oxidation state.
Which of the following metals corrodes readily in moist air ? [1980]- a)Gold
- b)Silver
- c)Nickel
- d)Iron
Correct answer is option 'D'. Can you explain this answer?
Which of the following metals corrodes readily in moist air ? [1980]
a)
Gold
b)
Silver
c)
Nickel
d)
Iron

|
Rajat Roy answered |
In moist air, corrosion of iron takes place in the form of rust (Fe2O3 + Fe(OH)3) . As iron is most reactive among all.
Which of the following oxidation states are the most characteristic for lead and tin respectively?- a)+ 2, + 4
- b)+ 4, + 4 [2007]
- c)+ 2, + 2
- d)+ 4, + 2
Correct answer is option 'A'. Can you explain this answer?
Which of the following oxidation states are the most characteristic for lead and tin respectively?
a)
+ 2, + 4
b)
+ 4, + 4 [2007]
c)
+ 2, + 2
d)
+ 4, + 2

|
Prasenjit Malik answered |
The oxidation states of lead and tin can vary depending on the compound they are present in. However, there are certain oxidation states that are more characteristic for these elements.
Lead:
- The most characteristic oxidation state for lead is +2. This means that lead can lose two electrons to form a 2+ cation. This oxidation state is commonly observed in lead compounds such as lead(II) oxide (PbO), lead(II) chloride (PbCl2), and lead(II) nitrate (Pb(NO3)2).
Tin:
- The most characteristic oxidation state for tin is +4. This means that tin can lose four electrons to form a 4+ cation. This oxidation state is commonly observed in tin compounds such as tin(IV) oxide (SnO2), tin(IV) chloride (SnCl4), and tin(IV) sulfate (Sn(SO4)2).
Explanation:
- The oxidation state of an element refers to the charge that element would have if all its bonds were ionic. It is a measure of the electron gain or loss of an atom in a compound.
- In the case of lead, it has a tendency to lose two electrons to achieve a stable configuration. This results in its most characteristic oxidation state of +2.
- Similarly, tin has a tendency to lose four electrons to achieve a stable configuration. This results in its most characteristic oxidation state of +4.
- It is important to note that lead and tin can also exhibit other oxidation states depending on the compound they are present in. For example, lead can also exist in the +4 oxidation state in compounds such as lead(IV) oxide (PbO2), while tin can also exist in the +2 oxidation state in compounds such as tin(II) chloride (SnCl2).
- However, the most characteristic oxidation states for lead and tin respectively are +2 and +4.
- Therefore, the correct answer is option A, which states that the most characteristic oxidation states for lead and tin are 2 and 4 respectively.
Lead:
- The most characteristic oxidation state for lead is +2. This means that lead can lose two electrons to form a 2+ cation. This oxidation state is commonly observed in lead compounds such as lead(II) oxide (PbO), lead(II) chloride (PbCl2), and lead(II) nitrate (Pb(NO3)2).
Tin:
- The most characteristic oxidation state for tin is +4. This means that tin can lose four electrons to form a 4+ cation. This oxidation state is commonly observed in tin compounds such as tin(IV) oxide (SnO2), tin(IV) chloride (SnCl4), and tin(IV) sulfate (Sn(SO4)2).
Explanation:
- The oxidation state of an element refers to the charge that element would have if all its bonds were ionic. It is a measure of the electron gain or loss of an atom in a compound.
- In the case of lead, it has a tendency to lose two electrons to achieve a stable configuration. This results in its most characteristic oxidation state of +2.
- Similarly, tin has a tendency to lose four electrons to achieve a stable configuration. This results in its most characteristic oxidation state of +4.
- It is important to note that lead and tin can also exhibit other oxidation states depending on the compound they are present in. For example, lead can also exist in the +4 oxidation state in compounds such as lead(IV) oxide (PbO2), while tin can also exist in the +2 oxidation state in compounds such as tin(II) chloride (SnCl2).
- However, the most characteristic oxidation states for lead and tin respectively are +2 and +4.
- Therefore, the correct answer is option A, which states that the most characteristic oxidation states for lead and tin are 2 and 4 respectively.
Which one of the elements with the following outer orbital configurations may exhibit the largest number of oxidation states? [2009]- a)3d54s1
- b)3d54s2
- c)3d24s2
- d)3d34s2
Correct answer is option 'B'. Can you explain this answer?
Which one of the elements with the following outer orbital configurations may exhibit the largest number of oxidation states? [2009]
a)
3d54s1
b)
3d54s2
c)
3d24s2
d)
3d34s2

|
Om Kumar answered |
Explanation:
Outer orbital configurations:
a) 3d5 4s1
b) 3d5 4s2
c) 3d2 4s2
d) 3d3 4s2
Understanding oxidation states:
Oxidation state refers to the number of electrons an atom loses, gains, or shares during a chemical reaction to form a compound. It is represented by a Roman numeral following the element symbol. The oxidation state depends on the number of valence electrons an atom has.
Determining oxidation states:
To determine the maximum number of oxidation states an element can exhibit, we need to consider the number of valence electrons.
Valence electrons:
Valence electrons are the electrons present in the outermost energy level of an atom. They are responsible for the chemical properties of an element.
Valence electrons in the given configurations:
a) 3d5 4s1 - This configuration has 1 valence electron.
b) 3d5 4s2 - This configuration has 2 valence electrons.
c) 3d2 4s2 - This configuration has 2 valence electrons.
d) 3d3 4s2 - This configuration has 2 valence electrons.
Analysis:
The element with the highest number of valence electrons has the potential to exhibit the largest number of oxidation states. In this case, option b) with 2 valence electrons has the highest number of valence electrons among the given configurations.
Reasoning:
The element in option b) with the configuration 3d5 4s2 is most likely to exhibit the largest number of oxidation states because it has the highest number of valence electrons, which allows for more possibilities of gaining or losing electrons during chemical reactions.
Conclusion:
Based on the number of valence electrons, the element with the outer orbital configuration 3d5 4s2 (option b) has the potential to exhibit the largest number of oxidation states among the given configurations.
Outer orbital configurations:
a) 3d5 4s1
b) 3d5 4s2
c) 3d2 4s2
d) 3d3 4s2
Understanding oxidation states:
Oxidation state refers to the number of electrons an atom loses, gains, or shares during a chemical reaction to form a compound. It is represented by a Roman numeral following the element symbol. The oxidation state depends on the number of valence electrons an atom has.
Determining oxidation states:
To determine the maximum number of oxidation states an element can exhibit, we need to consider the number of valence electrons.
Valence electrons:
Valence electrons are the electrons present in the outermost energy level of an atom. They are responsible for the chemical properties of an element.
Valence electrons in the given configurations:
a) 3d5 4s1 - This configuration has 1 valence electron.
b) 3d5 4s2 - This configuration has 2 valence electrons.
c) 3d2 4s2 - This configuration has 2 valence electrons.
d) 3d3 4s2 - This configuration has 2 valence electrons.
Analysis:
The element with the highest number of valence electrons has the potential to exhibit the largest number of oxidation states. In this case, option b) with 2 valence electrons has the highest number of valence electrons among the given configurations.
Reasoning:
The element in option b) with the configuration 3d5 4s2 is most likely to exhibit the largest number of oxidation states because it has the highest number of valence electrons, which allows for more possibilities of gaining or losing electrons during chemical reactions.
Conclusion:
Based on the number of valence electrons, the element with the outer orbital configuration 3d5 4s2 (option b) has the potential to exhibit the largest number of oxidation states among the given configurations.
The common oxidation states of Ti are [1994]- a)+ 2, + 3
- b)+ 3, + 4
- c)– 3, – 4
- d)+ 2, + 3, + 4
Correct answer is option 'D'. Can you explain this answer?
The common oxidation states of Ti are [1994]
a)
+ 2, + 3
b)
+ 3, + 4
c)
– 3, – 4
d)
+ 2, + 3, + 4

|
Shivani Tiwari answered |
The minimum oxidation state in transition metal is equal to the number of electrons in 4s shell and the maximum oxidation state is equal to the sum of the 4s and 3d electrons.
Ti 3d2 4s2 hence minimum oxidation state is +2 and maximum oxidation state is +4. Thus the common oxidation states of Ti are +2, +3 & +4
Ti 3d2 4s2 hence minimum oxidation state is +2 and maximum oxidation state is +4. Thus the common oxidation states of Ti are +2, +3 & +4
Prussian blue is formed when [1989]- a)Ferrous sulphate reacts with FeCl3
- b)Ferric chloride reacts with K4 [Fe(CN)6]
- c)Fer rous ammon ium sulphate reacts with FeCl3
- d)Ammonium sulphate reacts with FeCl3
Correct answer is option 'B'. Can you explain this answer?
Prussian blue is formed when [1989]
a)
Ferrous sulphate reacts with FeCl3
b)
Ferric chloride reacts with K4 [Fe(CN)6]
c)
Fer rous ammon ium sulphate reacts with FeCl3
d)
Ammonium sulphate reacts with FeCl3
|
|
Preethi Kulkarni answered |
Potassium ferrocyanide solution is added to Fe3+ ions in solution to give deep blue solution or precipitate.
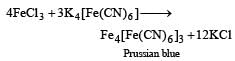
Which of the statements is not true? [2012]- a)On passing H2S through acidified K2Cr2O7 solution, a milky colour is observed.
- b)Na2Cr2O7 is preferred over K2Cr2O7 in volumetric analysis.
- c)K2Cr2O7 solution in acidic medium is orange.
- d)K2 Cr2O7 solution becomes yellow on increasing the pH beyond 7.
Correct answer is option 'B'. Can you explain this answer?
Which of the statements is not true? [2012]
a)
On passing H2S through acidified K2Cr2O7 solution, a milky colour is observed.
b)
Na2Cr2O7 is preferred over K2Cr2O7 in volumetric analysis.
c)
K2Cr2O7 solution in acidic medium is orange.
d)
K2 Cr2O7 solution becomes yellow on increasing the pH beyond 7.

|
Ruchi Chakraborty answered |
Na2Cr2O7 is hygroscopic.
Which of the following combines with Fe (II) ions to form a brown complex? [2000]- a)NO
- b)N2O
- c)N2O3
- d)N2O5
Correct answer is option 'A'. Can you explain this answer?
Which of the following combines with Fe (II) ions to form a brown complex? [2000]
a)
NO
b)
N2O
c)
N2O3
d)
N2O5

|
Mihir Patel answered |
Formation of Brown Complex with Fe (II) ions
Fe (II) ions can form a brown complex when they combine with certain ligands. The specific ligand that combines with Fe (II) ions to form a brown complex is NO (nitric oxide).
Explanation
- When Fe (II) ions react with NO, they form a complex known as iron nitrosyl complex.
- This complex is brown in color, giving the solution a brown appearance.
- The formation of this brown complex is due to the interaction between the Fe (II) ions and NO ligands.
Therefore, among the options provided, only NO (option A) can combine with Fe (II) ions to form a brown complex.
Fe (II) ions can form a brown complex when they combine with certain ligands. The specific ligand that combines with Fe (II) ions to form a brown complex is NO (nitric oxide).
Explanation
- When Fe (II) ions react with NO, they form a complex known as iron nitrosyl complex.
- This complex is brown in color, giving the solution a brown appearance.
- The formation of this brown complex is due to the interaction between the Fe (II) ions and NO ligands.
Therefore, among the options provided, only NO (option A) can combine with Fe (II) ions to form a brown complex.
Which of the following lanth anoidions is diamagnetic ? (At nos. Ce = 58, Sm = 62, Eu = 63, Yb = 70) [NEET 2013]- a)Sm2+
- b)Eu2+
- c)Yb2+
- d)Ce2+
Correct answer is option 'C'. Can you explain this answer?
Which of the following lanth anoidions is diamagnetic ? (At nos. Ce = 58, Sm = 62, Eu = 63, Yb = 70) [NEET 2013]
a)
Sm2+
b)
Eu2+
c)
Yb2+
d)
Ce2+
|
|
Rhea Iyer answered |
Diamagnetic materials are those that have all their electrons paired up in their atomic or molecular orbitals, resulting in no net magnetic moment. On the other hand, paramagnetic materials have unpaired electrons and exhibit a net magnetic moment.
In the given options, we need to identify the lanthanoid anion that is diamagnetic.
Let's analyze each option one by one:
a) Sm2: Samarium (Sm) has an atomic number of 62. In its neutral state, it has the electron configuration [Xe] 4f6 6s2. Sm2 would mean that two electrons are removed from the 6s orbital. The resulting electron configuration would be [Xe] 4f6. Since there are unpaired electrons present, Sm2 is paramagnetic and not diamagnetic.
b) Eu2: Europium (Eu) has an atomic number of 63. In its neutral state, it has the electron configuration [Xe] 4f7 6s2. Eu2 would mean that two electrons are removed from the 6s orbital. The resulting electron configuration would be [Xe] 4f7. Since there are unpaired electrons present, Eu2 is paramagnetic and not diamagnetic.
c) Yb2: Ytterbium (Yb) has an atomic number of 70. In its neutral state, it has the electron configuration [Xe] 4f14 6s2. Yb2 would mean that two electrons are removed from the 6s orbital. The resulting electron configuration would be [Xe] 4f14. Since all the orbitals are completely filled and there are no unpaired electrons, Yb2 is diamagnetic.
d) Ce2: Cerium (Ce) has an atomic number of 58. In its neutral state, it has the electron configuration [Xe] 4f1 5d1 6s2. Ce2 would mean that two electrons are removed from the 6s orbital. The resulting electron configuration would be [Xe] 4f1 5d1. Since there is an unpaired electron present in the 4f orbital, Ce2 is paramagnetic and not diamagnetic.
Therefore, the correct answer is option C, Yb2, as it is the only lanthanoid anion that has a completely filled f-orbital and no unpaired electrons, making it diamagnetic.
In the given options, we need to identify the lanthanoid anion that is diamagnetic.
Let's analyze each option one by one:
a) Sm2: Samarium (Sm) has an atomic number of 62. In its neutral state, it has the electron configuration [Xe] 4f6 6s2. Sm2 would mean that two electrons are removed from the 6s orbital. The resulting electron configuration would be [Xe] 4f6. Since there are unpaired electrons present, Sm2 is paramagnetic and not diamagnetic.
b) Eu2: Europium (Eu) has an atomic number of 63. In its neutral state, it has the electron configuration [Xe] 4f7 6s2. Eu2 would mean that two electrons are removed from the 6s orbital. The resulting electron configuration would be [Xe] 4f7. Since there are unpaired electrons present, Eu2 is paramagnetic and not diamagnetic.
c) Yb2: Ytterbium (Yb) has an atomic number of 70. In its neutral state, it has the electron configuration [Xe] 4f14 6s2. Yb2 would mean that two electrons are removed from the 6s orbital. The resulting electron configuration would be [Xe] 4f14. Since all the orbitals are completely filled and there are no unpaired electrons, Yb2 is diamagnetic.
d) Ce2: Cerium (Ce) has an atomic number of 58. In its neutral state, it has the electron configuration [Xe] 4f1 5d1 6s2. Ce2 would mean that two electrons are removed from the 6s orbital. The resulting electron configuration would be [Xe] 4f1 5d1. Since there is an unpaired electron present in the 4f orbital, Ce2 is paramagnetic and not diamagnetic.
Therefore, the correct answer is option C, Yb2, as it is the only lanthanoid anion that has a completely filled f-orbital and no unpaired electrons, making it diamagnetic.
The most durable metal plating on iron to protect against corrosion is [1994]- a)Nickel plating
- b)Tin plating
- c)Copper plating
- d)Zinc plating
Correct answer is option 'D'. Can you explain this answer?
The most durable metal plating on iron to protect against corrosion is [1994]
a)
Nickel plating
b)
Tin plating
c)
Copper plating
d)
Zinc plating

|
Aniket Chawla answered |
Galvanisation is the process of deposition of zinc metal on the surface of Fe to prevent it from rusting etc. Zinc forms a protective layer of basic carbonate (ZnCO3. Zn(OH)2) on it.
The electronic configuration of Cu (atomic number 29) is [1991]- a)1s2 , 2s2 2p6 , 3s2 3p6 , 4s2 3d9
- b)1s2 , 2s2 2p6 , 3s2 3p6 3d10 , 4s1
- c)1s2 , 2s2 2p6 , 3p2 3p6 , 4s2 4p6 , 5s2 5p1
- d)1s2 , 2s2 2p6 , 3p2 3p6 , 4s2 4p6 3d3
Correct answer is option 'B'. Can you explain this answer?
The electronic configuration of Cu (atomic number 29) is [1991]
a)
1s2 , 2s2 2p6 , 3s2 3p6 , 4s2 3d9
b)
1s2 , 2s2 2p6 , 3s2 3p6 3d10 , 4s1
c)
1s2 , 2s2 2p6 , 3p2 3p6 , 4s2 4p6 , 5s2 5p1
d)
1s2 , 2s2 2p6 , 3p2 3p6 , 4s2 4p6 3d3

|
Kavya Das answered |
The electronic configuration of an element represents the distribution of its electrons in different energy levels and orbitals. In the case of Copper (Cu), which has an atomic number of 29, the electronic configuration can be determined using the Aufbau principle and the rules for filling the orbitals.
According to the Aufbau principle, electrons fill the lowest energy orbitals first before moving to higher energy orbitals. The electronic configuration of Copper (Cu) can be determined by examining the filling order of the different orbitals.
The electronic configuration of Copper (Cu) is:
1s2, 2s2 2p6, 3s2 3p6, 3d10, 4s1
Let's break down the electronic configuration step by step:
1. Filling the 1s orbital:
The first two electrons of Copper (Cu) fill the 1s orbital, which can hold a maximum of two electrons.
2. Filling the 2s and 2p orbitals:
The next eight electrons fill the 2s and 2p orbitals. The 2s orbital can hold a maximum of two electrons, and the 2p orbital can hold a maximum of six electrons.
3. Filling the 3s and 3p orbitals:
The following ten electrons fill the 3s and 3p orbitals. The 3s orbital can hold a maximum of two electrons, and the 3p orbital can hold a maximum of six electrons.
4. Filling the 3d orbital:
The next ten electrons fill the 3d orbital. The 3d orbital can hold a maximum of ten electrons, and in the case of Copper (Cu), it has a configuration of 3d10.
5. Filling the 4s orbital:
The last electron of Copper (Cu) fills the 4s orbital, which can hold a maximum of two electrons.
Therefore, the complete electronic configuration of Copper (Cu) is:
1s2, 2s2 2p6, 3s2 3p6, 3d10, 4s1
This configuration follows the rules of filling the orbitals in order of increasing energy levels and adheres to the Aufbau principle.
According to the Aufbau principle, electrons fill the lowest energy orbitals first before moving to higher energy orbitals. The electronic configuration of Copper (Cu) can be determined by examining the filling order of the different orbitals.
The electronic configuration of Copper (Cu) is:
1s2, 2s2 2p6, 3s2 3p6, 3d10, 4s1
Let's break down the electronic configuration step by step:
1. Filling the 1s orbital:
The first two electrons of Copper (Cu) fill the 1s orbital, which can hold a maximum of two electrons.
2. Filling the 2s and 2p orbitals:
The next eight electrons fill the 2s and 2p orbitals. The 2s orbital can hold a maximum of two electrons, and the 2p orbital can hold a maximum of six electrons.
3. Filling the 3s and 3p orbitals:
The following ten electrons fill the 3s and 3p orbitals. The 3s orbital can hold a maximum of two electrons, and the 3p orbital can hold a maximum of six electrons.
4. Filling the 3d orbital:
The next ten electrons fill the 3d orbital. The 3d orbital can hold a maximum of ten electrons, and in the case of Copper (Cu), it has a configuration of 3d10.
5. Filling the 4s orbital:
The last electron of Copper (Cu) fills the 4s orbital, which can hold a maximum of two electrons.
Therefore, the complete electronic configuration of Copper (Cu) is:
1s2, 2s2 2p6, 3s2 3p6, 3d10, 4s1
This configuration follows the rules of filling the orbitals in order of increasing energy levels and adheres to the Aufbau principle.
Cuprous compounds such as CuCl, CuCN and CuSCN are the only salts stable in water due to- a)high hydration energy of Cu+ ions [1996]
- b)their inherent tendency not do disproportionate
- c)diamagnetic nature
- d)insolubility in water
Correct answer is option 'A'. Can you explain this answer?
Cuprous compounds such as CuCl, CuCN and CuSCN are the only salts stable in water due to
a)
high hydration energy of Cu+ ions [1996]
b)
their inherent tendency not do disproportionate
c)
diamagnetic nature
d)
insolubility in water

|
Abhishek Choudhary answered |
The smaller is the size of cation, the more is heat of hydration. Since hydration is exothermic process, thus heat evolves and hence system having lower energy are stable.
Identify the incorrect statement among the following: [2007]- a)Lanthanoid contraction is the accumulation of successive shrinkages.
- b)As a result of lanthanoid contraction, the properties of 4d series of the transition elements have no similarities with the 5d series of elements.
- c)Shielding power of 4f electron s is qui te weak.
- d)There is a decrease in the radii of the atoms or ions as one proceeds from La to Lu.
Correct answer is option 'B'. Can you explain this answer?
Identify the incorrect statement among the following: [2007]
a)
Lanthanoid contraction is the accumulation of successive shrinkages.
b)
As a result of lanthanoid contraction, the properties of 4d series of the transition elements have no similarities with the 5d series of elements.
c)
Shielding power of 4f electron s is qui te weak.
d)
There is a decrease in the radii of the atoms or ions as one proceeds from La to Lu.

|
Rohan Unni answered |
There is a steady decrease in the radii as the atomic number of the lanthanide elements increases. For every additional proton added in nucleus the corresponding electron goes to 4f subshell.
The shape of f -orbitals is very much diffused and they have poor shielding effect. The effective nuclear charge increases which causes the contraction in the size of electron charge cloud. This contraction in size is quite regular and known as Lanthanoid contraction.
Since the change in the ionic radii in the lanthanide series is very small, thus their chemical properties are similar.
The shape of f -orbitals is very much diffused and they have poor shielding effect. The effective nuclear charge increases which causes the contraction in the size of electron charge cloud. This contraction in size is quite regular and known as Lanthanoid contraction.
Since the change in the ionic radii in the lanthanide series is very small, thus their chemical properties are similar.
Qut of TiF62–, CoF63–, Cu2Cl2 and NiCl24– (Z of Ti = 22, Co = 27, Cu = 29, Ni = 28), the colourless species are: [2009]- a)Cu2Cl2 and NiCl24–
- b)TiF62–,and Cu2Cl2
- c)CoF63– ,and NiCl24–
- d)TiF62–,and CoF63–
Correct answer is option 'B'. Can you explain this answer?
Qut of TiF62–, CoF63–, Cu2Cl2 and NiCl24– (Z of Ti = 22, Co = 27, Cu = 29, Ni = 28), the colourless species are: [2009]
a)
Cu2Cl2 and NiCl24–
b)
TiF62–,and Cu2Cl2
c)
CoF63– ,and NiCl24–
d)
TiF62–,and CoF63–

|
Pankaj Banerjee answered |
The colour exhibited by transition metal ions is due to the presence of unpaired electrons in -orbitals which permits the - excitation of electrons.
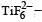 Ti is in + 4 O.S. ; 3d0 = colourless
Ti is in + 4 O.S. ; 3d0 = colourless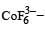 Co is in + 3 O.S ; 3d5 = coloured
Co is in + 3 O.S ; 3d5 = colouredIn Cu2Cl2– Cu is in +1 O.S. ; 3d10 – colourless
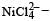 Ni is in + 2 O.S ; 3d8 – coloured
Ni is in + 2 O.S ; 3d8 – colouredWhich one of the following ions is the most stable in aqueous solution? [2007] (At.No. Ti = 22, V = 23, Cr = 24, Mn = 25)- a)V3+
- b)Ti3+
- c)Mn 3+
- d)Cr3+
Correct answer is option 'D'. Can you explain this answer?
Which one of the following ions is the most stable in aqueous solution? [2007] (At.No. Ti = 22, V = 23, Cr = 24, Mn = 25)
a)
V3+
b)
Ti3+
c)
Mn 3+
d)
Cr3+

|
Ishaan Menon answered |
For chromium ion + 3oxidation state is most stable.
Acidified K2Cr2O7 solution turns green when Na2SO3 is added to it. This is due to the formation of : [2011]- a)Cr2(SO4)3
- b)CrO42–
- c)Cr2(SO3)3
- d)CrSO4
Correct answer is option 'A'. Can you explain this answer?
Acidified K2Cr2O7 solution turns green when Na2SO3 is added to it. This is due to the formation of : [2011]
a)
Cr2(SO4)3
b)
CrO42–
c)
Cr2(SO3)3
d)
CrSO4

|
Akshat Chavan answered |
The green colour appears due to the formation of Cr+++ion

An acidic solution of 'X' does not give precipitate on passing H2S through it. 'X' gives white precipitate when NH4OH is added to it. The white precipitate dissolves in excess of NaOH solution.Pure 'X' fumes in air and dense white fumes are obtained when a glass rod dipped in NH4OH is put in the fumes. Compound 'X' can be [1999]- a)ZnCl2
- b)FeCl3
- c)AlCl3
- d)SnCl2
Correct answer is option 'A'. Can you explain this answer?
An acidic solution of 'X' does not give precipitate on passing H2S through it. 'X' gives white precipitate when NH4OH is added to it. The white precipitate dissolves in excess of NaOH solution.Pure 'X' fumes in air and dense white fumes are obtained when a glass rod dipped in NH4OH is put in the fumes. Compound 'X' can be [1999]
a)
ZnCl2
b)
FeCl3
c)
AlCl3
d)
SnCl2
|
|
Amar Das answered |
Identification of Compound X
Acidic Solution of X Does Not Give Precipitate on Passing H2S Through It
This indicates that there is no presence of sulfide ions in the compound X.
X Gives White Precipitate When NH4OH is Added to It
This indicates that the compound X is a metal cation that forms a white precipitate with hydroxide ions.
The White Precipitate Dissolves in Excess of NaOH Solution
This indicates that the white precipitate formed is an amphoteric hydroxide that dissolves in excess of a strong base due to the formation of a soluble complex.
Pure X Fumes in Air
This indicates that the compound X is volatile and can evaporate easily.
Dense White Fumes are Obtained When a Glass Rod Dipped in NH4OH is Put in the Fumes
This indicates that the compound X reacts with NH4OH to form a volatile compound that fumes in air.
Compound X Can Be ZnCl2, FeCl3, AlCl3, or SnCl2
ZnCl2 is the correct answer because it fits all the observations made. Zinc is a metal cation that forms a white precipitate with NH4OH, and the precipitate dissolves in excess NaOH to form a soluble complex. Zinc is also volatile and can fume in air, and it reacts with NH4OH to form a volatile compound that fumes in air. FeCl3 and AlCl3 both give a brown precipitate with H2S, while SnCl2 gives a black precipitate with H2S. Therefore, they do not fit the observation that X does not give a precipitate with H2S.
Acidic Solution of X Does Not Give Precipitate on Passing H2S Through It
This indicates that there is no presence of sulfide ions in the compound X.
X Gives White Precipitate When NH4OH is Added to It
This indicates that the compound X is a metal cation that forms a white precipitate with hydroxide ions.
The White Precipitate Dissolves in Excess of NaOH Solution
This indicates that the white precipitate formed is an amphoteric hydroxide that dissolves in excess of a strong base due to the formation of a soluble complex.
Pure X Fumes in Air
This indicates that the compound X is volatile and can evaporate easily.
Dense White Fumes are Obtained When a Glass Rod Dipped in NH4OH is Put in the Fumes
This indicates that the compound X reacts with NH4OH to form a volatile compound that fumes in air.
Compound X Can Be ZnCl2, FeCl3, AlCl3, or SnCl2
ZnCl2 is the correct answer because it fits all the observations made. Zinc is a metal cation that forms a white precipitate with NH4OH, and the precipitate dissolves in excess NaOH to form a soluble complex. Zinc is also volatile and can fume in air, and it reacts with NH4OH to form a volatile compound that fumes in air. FeCl3 and AlCl3 both give a brown precipitate with H2S, while SnCl2 gives a black precipitate with H2S. Therefore, they do not fit the observation that X does not give a precipitate with H2S.
The basic character of the transition metal monoxides follows the order [2003] (Atomic Nos.,Ti = 22, V = 23, Cr = 24, Fe = 26)- a)TiO > VO > CrO > FeO
- b)VO > CrO > TiO > FeO
- c)CrO > VO > FeO > TiO
- d)TiO > FeO > VO > CrO
Correct answer is option 'A'. Can you explain this answer?
The basic character of the transition metal monoxides follows the order [2003] (Atomic Nos.,Ti = 22, V = 23, Cr = 24, Fe = 26)
a)
TiO > VO > CrO > FeO
b)
VO > CrO > TiO > FeO
c)
CrO > VO > FeO > TiO
d)
TiO > FeO > VO > CrO

|
Gauri Khanna answered |
The basic character of the transition metal monoxides follows the order:
TiO < v2o="" />< cro="" />< />
This means that TiO is the least basic, while FeO is the most basic among the given transition metal monoxides.
TiO < v2o="" />< cro="" />< />
This means that TiO is the least basic, while FeO is the most basic among the given transition metal monoxides.
The lanthanide contraction is responsible for the fact that [1997] (Atomic numbers : Zr = 40, Y = 39, Nb = 41, Hf = 72, Zn = 30)- a)Zr and Y have about the same radius
- b)Zr and Nb have similar oxidation state
- c)Zr and Hf have about the same radius
- d)Zr and Zn have the same oxidation states
Correct answer is option 'C'. Can you explain this answer?
The lanthanide contraction is responsible for the fact that [1997] (Atomic numbers : Zr = 40, Y = 39, Nb = 41, Hf = 72, Zn = 30)
a)
Zr and Y have about the same radius
b)
Zr and Nb have similar oxidation state
c)
Zr and Hf have about the same radius
d)
Zr and Zn have the same oxidation states

|
Tejas Chavan answered |
We know that regular decrease in the size of the atoms and ions is called lanthanide contraction. In vertical column of transition elements there is a very small change in size and some times size is found same from second member to third member.The similarity in size of the atoms of Zr and Hf is evident due to the object of lanthanide contraction. Therefore Zr and Hf both have same radius 160 pm.
Actinides [1994]- a)Are all synthetic elements
- b)Include element 104
- c)Have any short lived isotopes
- d)Have variable valency
Correct answer is option 'D'. Can you explain this answer?
Actinides [1994]
a)
Are all synthetic elements
b)
Include element 104
c)
Have any short lived isotopes
d)
Have variable valency

|
Deepak Joshi answered |
Actinides have variable valency due to very small difference in energies of 5f, 6d and 7s orbitals. Actinides are the elements from atomic number 89 to 103.
The outer electronic configuration of Gd (At. No. 64) is: [NEET Kar. 2013]- a) 4f3 5d5 6s2
- b)4f8 5d0 6s2
- c) 4f7 5d1 6s2
- d)4f4 5d4 6s2
Correct answer is option 'C'. Can you explain this answer?
The outer electronic configuration of Gd (At. No. 64) is: [NEET Kar. 2013]
a)
4f3 5d5 6s2
b)
4f8 5d0 6s2
c)
4f7 5d1 6s2
d)
4f4 5d4 6s2

|
Tanisha Shah answered |
Answer of this question can be easily obtain from d and f block chapter
it is an element of lanthanoid series
Due to stability of half filled orbital answer is c
it is an element of lanthanoid series
Due to stability of half filled orbital answer is c
Four successive members of the first series of the transition metals are listed below. For which one of them the standard potential (E0M2+ /M) value has a positive sign? [2012 M]- a)Co (Z = 27)
- b)Ni (Z = 28)
- c)Cu (Z = 29)
- d)Fe (Z = 26)
Correct answer is option 'C'. Can you explain this answer?
Four successive members of the first series of the transition metals are listed below. For which one of them the standard potential (E0M2+ /M) value has a positive sign? [2012 M]
a)
Co (Z = 27)
b)
Ni (Z = 28)
c)
Cu (Z = 29)
d)
Fe (Z = 26)

|
Arpita Tiwari answered |
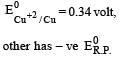
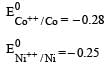
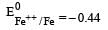
Which one of the following ionic species will impart colour to an aqueous solution? [1998]- a)Ti4+
- b)Cu+
- c)Zn2+
- d)Cr3+
Correct answer is option 'D'. Can you explain this answer?
Which one of the following ionic species will impart colour to an aqueous solution? [1998]
a)
Ti4+
b)
Cu+
c)
Zn2+
d)
Cr3+

|
Aditi Pandey answered |
Color is mainly due to excitation of electrons
ln Ti+4 there is no electron left for excitation
ln Cu 3d10 4s1 so after losing one electron all the remaining electron are paired similarly in case of
Zn 3d10 4s2 after losing 2 electrons are electrons are paired and no one is left for excitation
ln Ti+4 there is no electron left for excitation
ln Cu 3d10 4s1 so after losing one electron all the remaining electron are paired similarly in case of
Zn 3d10 4s2 after losing 2 electrons are electrons are paired and no one is left for excitation
Cinnabar is an ore of [1991]- a)Hg
- b)Cu
- c)Pb
- d)Zn
Correct answer is option 'A'. Can you explain this answer?
Cinnabar is an ore of [1991]
a)
Hg
b)
Cu
c)
Pb
d)
Zn

|
Nayanika Reddy answered |
Cinnabar (HgS) is an ore of Hg.
Chapter doubts & questions for The d and f-Block Elements - Chemistry 31 Years NEET Chapterwise Solved Papers 2025 is part of NEET exam preparation. The chapters have been prepared according to the NEET exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for NEET 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of The d and f-Block Elements - Chemistry 31 Years NEET Chapterwise Solved Papers in English & Hindi are available as part of NEET exam.
Download more important topics, notes, lectures and mock test series for NEET Exam by signing up for free.

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup on EduRev and stay on top of your study goals
10M+ students crushing their study goals daily