All Exams >
UPSC >
Old & New NCERTs for IAS Preparation (Must Read) >
All Questions
All questions of Class 10 NCERT Chemistry for UPSC CSE Exam
The acid present in curd is:a)Lactic acidb)Citric acidc)Oxalic acidd)Acetic acidCorrect answer is 'A'. Can you explain this answer?
|
|
Rahul Kapoor answered |
The acid present in curd is lactic acid.The increased acidity causes the milk proteins (casein) to tangle into solid masses, or curds. Milk that has been left to sour (raw milk alone or pasteurized milk with added lactic acid bacteria) will also naturally produce curds, and sour milk cheeses are produced this way.
Can you explain the answer of this question below:Select the substance which shows acidic behavior in its aqueous solution.- A:C6H12O6
- B:HCl
- C:C2H5OH
- D:Urea
The answer is b.
Select the substance which shows acidic behavior in its aqueous solution.
A:
C6H12O6
B:
HCl
C:
C2H5OH
D:
Urea
|
|
Alisha kapoor answered |
Acid is defined as the substance which dissociates and releases H+ ions in their aqueous states.
The equation for the dissociation of hydrochloric acid is given by:
HCl (aq.) gives H+ (aq.) + OH-(aq)
But molecules like alcohol and glucose and Urea do not produce H+ ions and thus are not considered as acids.
Name an acid which contains both oxygen and hydrogen?- a)Oxy acid
- b)Hydra acid
- c)Dilute acid
- d)Concentrated acid
Correct answer is option 'A'. Can you explain this answer?
Name an acid which contains both oxygen and hydrogen?
a)
Oxy acid
b)
Hydra acid
c)
Dilute acid
d)
Concentrated acid

|
Ameya Rane answered |
Oxy acid is an acid which contains both oxygen and hydrogen.
An oxyacid is an acid that contains an oxygen atom bonded to a hydrogen atom and at least one other element.
An oxyacid has the general structure X-O-H.
Which of the following does not conduct electricity?- a)Sodium hydroxide
- b)Rain water
- c)Hydrochloric acid
- d)Distilled water
Correct answer is option 'D'. Can you explain this answer?
Which of the following does not conduct electricity?
a)
Sodium hydroxide
b)
Rain water
c)
Hydrochloric acid
d)
Distilled water
|
|
Gaurav Kumar answered |
Distilled water do not conduct electricity. The reason is that a liquid conducts electricity is by the positively or negatively charged ions that are actually moving from one of the electrodes to the other, carrying charge (electricity) with them.
Lime water is- a)CaO
- b)Ca(OH)2
- c)CaCO3
- d)CaCI2
Correct answer is option 'B'. Can you explain this answer?
Lime water is
a)
CaO
b)
Ca(OH)2
c)
CaCO3
d)
CaCI2
|
|
Kiran Mehta answered |
Limewater is the common name for a dilute aqueous solution of calcium hydroxide.
Calcium hydroxide, Ca(OH)2, is sparsely soluble at room temperature in water (1.5 g/L at 25 °C). It is basic in nature with a pH of 12.4.
Calcium hydroxide, Ca(OH)2, is sparsely soluble at room temperature in water (1.5 g/L at 25 °C). It is basic in nature with a pH of 12.4.
Which of the property is not shown by bases?- a)Bases dissociate in water to give OH- ions
- b)Bases are soapy in touch
- c)Bases turn blue litmus red
- d)Bases are bitter in taste
Correct answer is option 'C'. Can you explain this answer?
Which of the property is not shown by bases?
a)
Bases dissociate in water to give OH- ions
b)
Bases are soapy in touch
c)
Bases turn blue litmus red
d)
Bases are bitter in taste
|
|
Arun Sharma answered |
Blue litmus paper is mainly used to test acids. It will turn red when it comes into contact with an acid. If it comes into contact with a substance that is basic or neutral, it will remain blue.
Acids when dissolved in water produce:
- a)Hydrogen and hydroxide ions
- b)Hydroxide ions
- c)Hydride ions
- d)Hydronium ions
Correct answer is option 'D'. Can you explain this answer?
Acids when dissolved in water produce:
a)
Hydrogen and hydroxide ions
b)
Hydroxide ions
c)
Hydride ions
d)
Hydronium ions

|
Snehal Sen answered |
Most acids release H+ ions in water, which combines with water molecule to produce hydronium (H3O+) ion.
Eg.
HCl(g)+H2O(l)→Cl−(aq)+H3O+(aq)
Fe2O3 + 2Al → Al2O3 + 2Fe
The above reaction is an example of a:- a)Decomposition reaction
- b)Combination reaction
- c)Displacement reaction
- d)Single displacement reaction
Correct answer is option 'C'. Can you explain this answer?
Fe2O3 + 2Al → Al2O3 + 2Fe
The above reaction is an example of a:
The above reaction is an example of a:
a)
Decomposition reaction
b)
Combination reaction
c)
Displacement reaction
d)
Single displacement reaction

|
Anushka Chopra answered |
The given equation is a displacement reaction in which Fe of Fe2O3 has been displaced by Al. Hence, (c) is the correct option.
Can you explain the answer of this question below:Which of the following acid is a weak acid?- A:HCl
- B:H2SO4
- C:HNO3
- D:CH3COOH
The answer is d.
Which of the following acid is a weak acid?
A:
HCl
B:
H2SO4
C:
HNO3
D:
CH3COOH

|
Naina Chopra answered |
Acids like acetic acid ( CH3COOH ) dissociates partially or incompletely, releasing only some of its hydrogen atoms into the solution. Hence they are weak acid.
How many elements were known when Mendeleev started his work on his periodic table?- a)63
- b)55
- c)69
- d)45
Correct answer is option 'A'. Can you explain this answer?
How many elements were known when Mendeleev started his work on his periodic table?
a)
63
b)
55
c)
69
d)
45

|
Deepesh Singh Sengar. answered |
There is 63 element because Mendel put element according to their mass volumes
upvote my answer
upvote my answer
Which one will not change from red litmus to blue?a) NaClb) HClc) KOHd) LiOHCorrect answer is option 'B'. Can you explain this answer?
b) HCl
c) KOH
d) LiOH
Correct answer is option 'B'. Can you explain this answer?

|
Tarun Mehra answered |
Explanation: Since HCl is a base , so it turns red litmus to blue.
So option B is a correct answer.
Which of the following base is used in the manufacture of bleaching powder?- a)Magnesium hydroxide
- b)Sodium hydroxide
- c)Potassium hydroxide
- d)Calcium hydroxide
Correct answer is option 'D'. Can you explain this answer?
Which of the following base is used in the manufacture of bleaching powder?
a)
Magnesium hydroxide
b)
Sodium hydroxide
c)
Potassium hydroxide
d)
Calcium hydroxide

|
Aditya Kumar answered |
Because.... ca(OH)2+ cl2= caocl2 + h2o that's why
A strip of copper was placed in a beaker containing zinc sulphate solution. On observing the strip next day, it was noticed that:- a)The colour of the copper strip changed.
- b)The copper strip remained as it was.
- c)The copper strip became thicker.
- d)The copper strip became thinner.
Correct answer is option 'B'. Can you explain this answer?
A strip of copper was placed in a beaker containing zinc sulphate solution. On observing the strip next day, it was noticed that:
a)
The colour of the copper strip changed.
b)
The copper strip remained as it was.
c)
The copper strip became thicker.
d)
The copper strip became thinner.
|
|
Khusboo bhatia answered |
A single replacement reaction is one in which a more reactive element displaces a less reactive element from its salt solution.
But copper can not lose electrons easily as compared to zinc and do not result in any reaction.
Thus there will no color change as no chemical reaction take place between the two.
The best conductor of electricity among the following is:- a)Hg (Mercury)
- b)Al (Aluminum)
- c)Ag (Silver)
- d)Au (Gold)
Correct answer is option 'C'. Can you explain this answer?
The best conductor of electricity among the following is:
a)
Hg (Mercury)
b)
Al (Aluminum)
c)
Ag (Silver)
d)
Au (Gold)
|
|
Avinash Patel answered |
Metals are good conductors of electricity. silver is found in in pure form from the earth. so In the given options all are metals. Among them silver is the best conductor of electricity after second best conductor of electricity is gold.
Metal which is a poor conductor of heat is:- a)Zinc
- b)Lead
- c)Gold
- d)Copper
Correct answer is option 'B'. Can you explain this answer?
Metal which is a poor conductor of heat is:
a)
Zinc
b)
Lead
c)
Gold
d)
Copper
|
|
Anjana Khatri answered |
Although metals are supposed to be good conductors of electricity and heat,metals like mercury, lead, alloys of iron and chromium, titanium and stainless steel are poor conductors when compared to silver, copper and gold.
Among the following metals, the metal that can displace zinc from zinc sulphate solution is:- a)Magnesium
- b)Copper
- c)Lead
- d)Silver
Correct answer is option 'A'. Can you explain this answer?
Among the following metals, the metal that can displace zinc from zinc sulphate solution is:
a)
Magnesium
b)
Copper
c)
Lead
d)
Silver
|
|
Arun Sharma answered |
Magnesium is more reactive than zinc therefore it can displace zinc from its solution.
Identify ‘X’ in the reaction: 2HCl + CuO → X + H2O- a)CuCl
- b)Cu(OH)2
- c)CuCl2
- d)HOCl
Correct answer is option 'C'. Can you explain this answer?
Identify ‘X’ in the reaction: 2HCl + CuO → X + H2O
a)
CuCl
b)
Cu(OH)2
c)
CuCl2
d)
HOCl
|
|
Vikram Kapoor answered |
When copper oxide and dilute hydrochloric acid are mixed the blue green solution is formed.
The reaction is :-
CuO + 2HCl → CuCl2 + H2O
The reaction is :-
CuO + 2HCl → CuCl2 + H2O
The colours of aqueous solutions of CuSO4 and FeSO4 as observed in the laboratory are:- a)Dark blue and pale green respectively.
- b)Dark blue and dark green respectively.
- c)Pale green and light blue respectively.
- d)Light blue and dark green respectively.
Correct answer is option 'A'. Can you explain this answer?
The colours of aqueous solutions of CuSO4 and FeSO4 as observed in the laboratory are:
a)
Dark blue and pale green respectively.
b)
Dark blue and dark green respectively.
c)
Pale green and light blue respectively.
d)
Light blue and dark green respectively.

|
Riya Verma answered |
Cu (2+)is of dark blue colour while Fe(2+) is of light green colour .So ,the colour of CuSO4 and Fe SO4 will be dark blue and pale green......
The second most abundant metal present in the crust of the earth is– - a)Ca
- b)Al
- c)Cu
- d)Fe
Correct answer is option 'D'. Can you explain this answer?
The second most abundant metal present in the crust of the earth is–
a)
Ca
b)
Al
c)
Cu
d)
Fe
|
|
Gaurav Kumar answered |
Iron is the second most abundant Metal in the earth's crust. It is found in the molten form inside the crust of the earth. In the presence of air and moisture, it forms it's oxide but the alloy of steel is widely used as pipes, it is unreactive in nature.
The natural source of Acetic acid is:- a)Curd
- b)Oranges
- c)Tomatoes
- d)Vinegar
Correct answer is option 'D'. Can you explain this answer?
The natural source of Acetic acid is:
a)
Curd
b)
Oranges
c)
Tomatoes
d)
Vinegar
|
|
Krishna Iyer answered |
Option A: Lactic acid in curd
Option B: Citrus acid in Oranges
Option C: Oxalic acid is found in tomatoes
Option D: Acetic acid is found in vinegar
Option B: Citrus acid in Oranges
Option C: Oxalic acid is found in tomatoes
Option D: Acetic acid is found in vinegar
Thus, option D is correct.
Which of the following metals comes above zinc in reactivity series?
- a)Silver
- b)Copper
- c)Aluminium
- d)Iron
Correct answer is option 'C'. Can you explain this answer?
Which of the following metals comes above zinc in reactivity series?
a)
Silver
b)
Copper
c)
Aluminium
d)
Iron
|
|
Karthik murthy answered |
Reactivity Series:
The reactivity series is a list of metals arranged in order of their decreasing reactivity. The most reactive metal is placed at the top and the least reactive metal is placed at the bottom of the series.
The reactivity series of metals is as follows:
Potassium > Sodium > Calcium > Magnesium > Aluminium > Zinc > Iron > Lead > Copper > Silver > Gold
Explanation:
Aluminium is more reactive than zinc and is placed above zinc in the reactivity series. This is because aluminium has a stronger tendency than zinc to lose electrons and form positive ions.
When aluminium is exposed to air, it reacts with oxygen to form a layer of aluminium oxide. This layer of oxide is very thin and is also very stable. It prevents further reaction of aluminium with oxygen and protects the metal from corrosion.
Zinc, on the other hand, reacts with oxygen in the air to form zinc oxide. However, the layer of oxide formed on zinc is not as stable as the layer of oxide formed on aluminium. Therefore, zinc is more susceptible to corrosion than aluminium.
Conclusion:
In conclusion, aluminium is more reactive than zinc and is placed above zinc in the reactivity series. This means that aluminium can displace zinc from its compounds in a chemical reaction, but zinc cannot displace aluminium from its compounds.
The reactivity series is a list of metals arranged in order of their decreasing reactivity. The most reactive metal is placed at the top and the least reactive metal is placed at the bottom of the series.
The reactivity series of metals is as follows:
Potassium > Sodium > Calcium > Magnesium > Aluminium > Zinc > Iron > Lead > Copper > Silver > Gold
Explanation:
Aluminium is more reactive than zinc and is placed above zinc in the reactivity series. This is because aluminium has a stronger tendency than zinc to lose electrons and form positive ions.
When aluminium is exposed to air, it reacts with oxygen to form a layer of aluminium oxide. This layer of oxide is very thin and is also very stable. It prevents further reaction of aluminium with oxygen and protects the metal from corrosion.
Zinc, on the other hand, reacts with oxygen in the air to form zinc oxide. However, the layer of oxide formed on zinc is not as stable as the layer of oxide formed on aluminium. Therefore, zinc is more susceptible to corrosion than aluminium.
Conclusion:
In conclusion, aluminium is more reactive than zinc and is placed above zinc in the reactivity series. This means that aluminium can displace zinc from its compounds in a chemical reaction, but zinc cannot displace aluminium from its compounds.
Identify the amphoteric oxide from the following:- a)MgO
- b)Na2O
- c)Al2O3
- d)K2O
Correct answer is option 'C'. Can you explain this answer?
Identify the amphoteric oxide from the following:
a)
MgO
b)
Na2O
c)
Al2O3
d)
K2O
|
|
Arun Sharma answered |
Amphoteric metal oxides react with both acids as well as bases to produce salt and water.
Aluminium oxide is an amphoteric oxide because it reacts with acids as well as bases and form salts and water.
Al2O3 + 6HCl → 2AlCl3 + 3H2O (Basic nature)
Aluminium Hydrochloric Aluminium Water
oxide acid chloride
Al2O3 + 2NaOH → 2NaAlO2 + H2O (Acidic nature)
Aluminium Sodium Sodium Water
oxide Hydroxide aluminate
All metals exist as solid at room temperature except the metal ‘X’. Name the metal “X’.- a)Tungsten
- b)Mercury
- c)Gold
- d)Potassium
Correct answer is option 'B'. Can you explain this answer?
All metals exist as solid at room temperature except the metal ‘X’. Name the metal “X’.
a)
Tungsten
b)
Mercury
c)
Gold
d)
Potassium
|
|
Ananya Das answered |
It is the only metal that is liquid at room temperature. It has the lowest melting point and boiling point of any other metal. It has low thermal conductivity, and a quite low electrical conductivity. It is the only metal that doesn’t form diatomic molecules in the gaseous phase.
Which of the following is an example of displacement reaction?- a)4 Na+ O2 → 2 Na2O
- b)2 Cu + O2 → 2 CuO
- c)Mg + 2 HCl → MgCl2 + H2
- d)N2 + 3 H2 → 2 NH3
Correct answer is option 'C'. Can you explain this answer?
Which of the following is an example of displacement reaction?
a)
4 Na+ O2 → 2 Na2O
b)
2 Cu + O2 → 2 CuO
c)
Mg + 2 HCl → MgCl2 + H2
d)
N2 + 3 H2 → 2 NH3
|
|
Pooja Shah answered |
It is a single replacement reaction.
Mg + 2HCl → MgCl2 + H2
Mg + 2HCl → MgCl2 + H2
Which metal is present in Calcium Hydroxide?- a)C
- b)O
- c)Ca
- d)H
Correct answer is option 'C'. Can you explain this answer?
Which metal is present in Calcium Hydroxide?
a)
C
b)
O
c)
Ca
d)
H
|
|
Amit Sharma answered |
Calcium hydroxide Ca ( OH) 2 , has calcium ( Ca) which is a metal.
The colour of phenolphthalein in acids is:- a)Colourless
- b)Red
- c)Pink
- d)Blue
Correct answer is option 'A'. Can you explain this answer?
The colour of phenolphthalein in acids is:
a)
Colourless
b)
Red
c)
Pink
d)
Blue
|
|
Krishna Iyer answered |
Phenolphthalein is often used as an indicator in acid–base titrations. For this application, it turns colourless in acidic solutions and magenta in basic solutions.
Name an element which is common to all acids?- a)Sulphur
- b)Chlorine
- c) Nitrogen
- d)Hydrogen
Correct answer is option 'D'. Can you explain this answer?
Name an element which is common to all acids?
a)
Sulphur
b)
Chlorine
c)
Nitrogen
d)
Hydrogen

|
Tarun Mehra answered |
An element which is common to all acids is Hydrogen .
So opttion D is correct option.
Find the odd one out:- a)Neutral salt : NaCl
- b)Acid salt : CuSO4.5H2O
- c) Basic salt: CuCO3.Cu(OH)2
- d)Nonhydrated salt: KNO3
Correct answer is option 'B'. Can you explain this answer?
Find the odd one out:
a)
Neutral salt : NaCl
b)
Acid salt : CuSO4.5H2O
c)
Basic salt: CuCO3.Cu(OH)2
d)
Nonhydrated salt: KNO3

|
Ashish Choudhary answered |
Explanation: CuSO4.5H20 is a hydrated salt. An example of acid salt is NaHCO3.
So option B is the correct answer.
Which of the following non-metal is good conductor of electricity?- a)Graphite
- b)Phosphorus
- c)Hydrogen
- d)Bromine
Correct answer is option 'A'. Can you explain this answer?
Which of the following non-metal is good conductor of electricity?
a)
Graphite
b)
Phosphorus
c)
Hydrogen
d)
Bromine
|
|
Gaurav Kumar answered |
Carbon, in the form of Graphite is a good conductor of electricity. It conducts heat and electricity like a metal or a metalloid.
The Law of Octaves was applicable only upto element ________.- a)Sodium
- b)Calcium
- c)Zinc
- d)Copper
Correct answer is option 'B'. Can you explain this answer?
The Law of Octaves was applicable only upto element ________.
a)
Sodium
b)
Calcium
c)
Zinc
d)
Copper
|
|
Vikram Kapoor answered |
The major limitations of Newlands' law of octaves were :
It was applicable to only lighter elements having atomic masses upto 40 u, i.e., upto calcium. After calcium, the first and the eighth element did not have similar properties. For example chromium (Cr) and yttrium (Y) are the first and the eighth element placed in the same column but they have entirely different properties.
It was applicable to only lighter elements having atomic masses upto 40 u, i.e., upto calcium. After calcium, the first and the eighth element did not have similar properties. For example chromium (Cr) and yttrium (Y) are the first and the eighth element placed in the same column but they have entirely different properties.
The colour of anhydrous copper sulphate is:- a)White
- b)Blue
- c)Red
- d)Yellow
Correct answer is option 'A'. Can you explain this answer?
The colour of anhydrous copper sulphate is:
a)
White
b)
Blue
c)
Red
d)
Yellow
|
|
Rahul Kapoor answered |
The answer is b.
The crystals of hydrated copper sulphate salt are blue in colour. When heated, the salt loses its water of crystallization and turns white.
Which of the following is not an organic acid?- a)Acetic acid
- b)Sulphuric acid
- c)Tartaric acid
- d)Citric acid
Correct answer is option 'B'. Can you explain this answer?
Which of the following is not an organic acid?
a)
Acetic acid
b)
Sulphuric acid
c)
Tartaric acid
d)
Citric acid
|
|
Amit Kumar answered |
Sulfuric acid is inorganic because it doesn't contain carbon atoms bonded to oxygen, nitrogen and hydrogen atoms.
Carbon dioxide is an example of:- a)Amphoteric oxide
- b)Acidic oxide
- c)Basic oxide
- d)Neutral oxide
Correct answer is option 'B'. Can you explain this answer?
Carbon dioxide is an example of:
a)
Amphoteric oxide
b)
Acidic oxide
c)
Basic oxide
d)
Neutral oxide
|
|
Gaurav Kumar answered |
Acid oxides is a complex chemical substance oxides, which form a salt with the chemical reactions with bases or basic oxides and do not react with acidic oxides.
Examples of acidic oxides can be:
CO2 (all known carbon dioxide), P2O5 - oxide of phosphorus (formed in air if burns white phosphorus), SO3 - oxide of sulfur (VI) is a substance used for sulfuric acid.
Examples of acidic oxides can be:
CO2 (all known carbon dioxide), P2O5 - oxide of phosphorus (formed in air if burns white phosphorus), SO3 - oxide of sulfur (VI) is a substance used for sulfuric acid.
Which one is more metallic element ?- a)Na
- b)Mg
- c)Al
- d)Si
Correct answer is option 'A'. Can you explain this answer?
Which one is more metallic element ?
a)
Na
b)
Mg
c)
Al
d)
Si
|
|
Diya Sharma answered |
Because it more fastly or quickly loss it's electron.
P, Q, R are elements of Dobereiner’s triads. If the atomic mass of P is 7 and that of Q is 23, What will be the atomic mass of R?- a)15.0
- b)40.0
- c)30.0
- d)39.0
Correct answer is option 'D'. Can you explain this answer?
P, Q, R are elements of Dobereiner’s triads. If the atomic mass of P is 7 and that of Q is 23, What will be the atomic mass of R?
a)
15.0
b)
40.0
c)
30.0
d)
39.0
|
|
Gaurav Kumar answered |
In a Dobereiner's traid, the atomic mass of the middle element is roughly the average of the atomic mass of the other 2 elements. Thus,
Atomic mass of Q = (at. mass of P+ at. mass of R) /2
⇒ 23 = 7+ m(R) /2
⇒ 23*2 = 7+ m(R)
⇒ 46 -7 = m(R)
⇒ atomic mass of R = 39
Atomic mass of Q = (at. mass of P+ at. mass of R) /2
⇒ 23 = 7+ m(R) /2
⇒ 23*2 = 7+ m(R)
⇒ 46 -7 = m(R)
⇒ atomic mass of R = 39
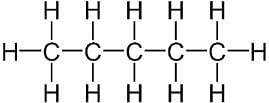
The number of C-H bonds in ethane(C2H6) molecule are:
- a)10
- b)8
- c)6
- d)4
Correct answer is option 'C'. Can you explain this answer?
The number of C-H bonds in ethane(C2H6) molecule are:
a)
10
b)
8
c)
6
d)
4
|
|
Naina Sharma answered |
The structure of the ethane molecule can be represented as:
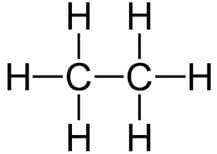
There are six C-H bonds and one C-C bond . So, Total 6 convalent bond between C-H

There are six C-H bonds and one C-C bond . So, Total 6 convalent bond between C-H
Which of the following metals does not displace H2 gas from dilute HCl or dilute H2SO4?- a)Mg
- b)Cu
- c)Zn
- d)Al
Correct answer is option 'B'. Can you explain this answer?
Which of the following metals does not displace H2 gas from dilute HCl or dilute H2SO4?
a)
Mg
b)
Cu
c)
Zn
d)
Al
|
|
Pooja Shah answered |
Copper doesn't react with dilute sulphuric acid, Liberating hydrogen because copper is lower in the electromotive series than hydrogen. A more reactive element can displace a less reactive element from its salt solution.
A lustrous non-metal is:- a)Iodine
- b)Bromine
- c)Chlorine
- d)Sulphur
Correct answer is option 'A'. Can you explain this answer?
A lustrous non-metal is:
a)
Iodine
b)
Bromine
c)
Chlorine
d)
Sulphur
|
|
Pooja Shah answered |
Non - metals do not have luster. They do not reflect light from their surface ( except iodine and diamond) . So, here a lustrous now Metal is iodine.
How many elements are placed in lanthanide and actinide series?- a)57, 89
- b)14, 14
- c)89, 57
- d)14, 16
Correct answer is option 'B'. Can you explain this answer?
How many elements are placed in lanthanide and actinide series?
a)
57, 89
b)
14, 14
c)
89, 57
d)
14, 16
|
|
Neha Patel answered |
14 elements
The same holds for the actinide series that runs from atomic number 90 through to number 103, again 14 elements. Thus, as you move from thorium (Th) at element number 90, you begin to fill up the 5f sublevel and continue to fill up the 5f sublevel until you finish the actinide series at lawrencium (Lr).
Identify the type of reaction: HCl + NaOH → NaCl + H2O- a)Combination reaction
- b)Double decomposition reaction
- c)Decomposition reaction
- d)Neutralisation reaction
Correct answer is option 'D'. Can you explain this answer?
Identify the type of reaction: HCl + NaOH → NaCl + H2O
a)
Combination reaction
b)
Double decomposition reaction
c)
Decomposition reaction
d)
Neutralisation reaction
|
|
Krishna Iyer answered |
Reaction of a strong acid with strong base is called neutralization reaction which produces salt and water,
HCl + NaOH → NaCl + H2O
This equation is already balanced.
Which gas causes the bread or cake to rise making them soft and spongy?- a)CO2
- b)O2
- c)CO
- d)HCl
Correct answer is option 'A'. Can you explain this answer?
Which gas causes the bread or cake to rise making them soft and spongy?
a)
CO2
b)
O2
c)
CO
d)
HCl
|
|
Vikram Kapoor answered |
Carbon dioxide is released when baking soda is added to cake and this makes the cake to rise making them soft and spongy.
Structural formula of ethyne is- a)
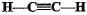
- b)
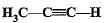
- c)
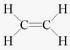
- d)
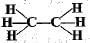
Correct answer is option 'A'. Can you explain this answer?
Structural formula of ethyne is
a)
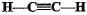
b)
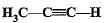
c)

d)
|
|
Puneet mukherjee answered |
'Eth' tells that there are 2 Carbon atoms and 'yne' tells that there is a triple bond.
Which of the following is not observed when aluminium is added to a solution of copper sulphate?- a)Final solution is light green.
- b)Solution is blue in the beginning.
- c)The brown mass is deposited on aluminium.
- d)Final solution is colourless.
Correct answer is option 'A'. Can you explain this answer?
Which of the following is not observed when aluminium is added to a solution of copper sulphate?
a)
Final solution is light green.
b)
Solution is blue in the beginning.
c)
The brown mass is deposited on aluminium.
d)
Final solution is colourless.
|
|
Rajiv Gupta answered |
When aluminum is added to copper sulphate solution, displacement reaction takes place. This means , aluminum being more reactive than copper..displaces it from its salt solution. ... The colour of the solution will change from blue to transparent and copper will be observed as a layer on the Aluminium piece.
The lustre of a metal is due to:- a)Its high density
- b)Presence of free electrons
- c)Its high polishing
- d)Its chemical inertness
Correct answer is option 'B'. Can you explain this answer?
The lustre of a metal is due to:
a)
Its high density
b)
Presence of free electrons
c)
Its high polishing
d)
Its chemical inertness
|
|
Rahul Kapoor answered |
THe lustre of a metal is due to presence of free electrons.
It has to do with the way light and electrons on the surface of metals interact. The outer electrons in a metal are almost not bound to any individual atom, thus are relatively free, and are concentrated on the surface. These electrons (electron density) tend to oscillate at a collective frequency.
As they oscillate they prevent any incoming light from entering the metal or passing through it. Consequently, the incident light is reflected back. Although a lot of materials also reflect, only those with a lot of nearly free electrons can reflect huge amount of light.
It is the intensity of this reflected light reaching our eyes that make it appear lustrous.
What happens when non-metals react with water?a) Hydrogen gas is formedb) Carbon dioxide gas is formedc) Non-metals do not react with waterd) None of these.Correct answer is option 'C'. Can you explain this answer?
b) Carbon dioxide gas is formed
c) Non-metals do not react with water
d) None of these.
Correct answer is option 'C'. Can you explain this answer?
|
|
Pooja Shah answered |
Non - metals do not react with water to evolve hydrogen gas. ( no reaction)
Which one of the following metal reacts vigorously with oxygen and water?
- a)Sodium
- b)Iron
- c)Calcium
- d)Magnesium
Correct answer is option 'A'. Can you explain this answer?
Which one of the following metal reacts vigorously with oxygen and water?
a)
Sodium
b)
Iron
c)
Calcium
d)
Magnesium
|
|
Ananya Das answered |
Sodium metal reacts vigorously with oxygen and water.
MCQ (Multiple Choice Questions) or Practice Quiz with solutions of Chapter - "Periodic Classification of Elements" of Class 10 Science, the questions are available for practice Q. According to IUPAC recommendations, the number of groups in the long form of the periodic table is :-- a)7
- b)8
- c)16
- d)18
Correct answer is option 'D'. Can you explain this answer?
MCQ (Multiple Choice Questions) or Practice Quiz with solutions of Chapter - "Periodic Classification of Elements" of Class 10 Science, the questions are available for practice
Q. According to IUPAC recommendations, the number of groups in the long form of the periodic table is :-
a)
7
b)
8
c)
16
d)
18
|
|
Krishna Iyer answered |
In chemistry, a group (also known as a family) is a column of elements in the periodic table of the chemical elements. There are 18 numbered groups in the periodic table.
How many triads could Dobereiner identify from the... more elements known at that time?a) 3b) 5c) 4d) 2Correct answer is option 'A'. Can you explain this answer?
|
|
Pooja Shah answered |
In 1817 a German chemist Johann Wolfgang Döbereiner arranged the elements with similar properties into groups.
Some groups were identified having three elements each. So he called these groups ‘triads’. Only three triads could be identified from the elements discovered at that time.
Chapter doubts & questions for Class 10 NCERT Chemistry - Old & New NCERTs for IAS Preparation (Must Read) 2025 is part of UPSC CSE exam preparation. The chapters have been prepared according to the UPSC CSE exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for UPSC CSE 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Class 10 NCERT Chemistry - Old & New NCERTs for IAS Preparation (Must Read) in English & Hindi are available as part of UPSC CSE exam.
Download more important topics, notes, lectures and mock test series for UPSC CSE Exam by signing up for free.
Old & New NCERTs for IAS Preparation (Must Read)
3 videos|706 docs|517 tests
|
Related UPSC CSE Content
Signup to see your scores go up within 7 days!
Study with 1000+ FREE Docs, Videos & Tests
10M+ students study on EduRev

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Forgot Password
OR
Signup to see your scores
go up within 7 days!
Access 1000+ FREE Docs, Videos and Tests
Takes less than 10 seconds to signup