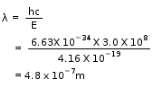All Exams >
NEET >
Topic-wise MCQ Tests for NEET >
All Questions
All questions of Structure of Atom for NEET Exam
Direction (Q. Nos. 12 and 13) This section contains a paragraph, wach describing theory, experiments, data etc. three Questions related to paragraph have been given.Each question have only one correct answer among the four given ptions (a),(b),(c),(d)A hypothetical electromagnetic wave is pictured here.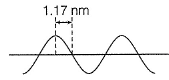 Q. Energy associated with this wave is
Q. Energy associated with this wave is- a)4.24 x 10-19J
- b)2.12 x 10-19J
- c)1.06 x 10-19J
- d)8.49 x 10-19J
Correct answer is option 'A'. Can you explain this answer?
Direction (Q. Nos. 12 and 13) This section contains a paragraph, wach describing theory, experiments, data etc. three Questions related to paragraph have been given.Each question have only one correct answer among the four given ptions (a),(b),(c),(d)
A hypothetical electromagnetic wave is pictured here.
Q. Energy associated with this wave is
a)
4.24 x 10-19J
b)
2.12 x 10-19J
c)
1.06 x 10-19J
d)
8.49 x 10-19J
|
|
Hansa Sharma answered |
A to E makes one complete wave.
The number of radial nodes for 3p orbital is __________.- a)3
- b)4
- c)2
- d)1
Correct answer is 'D'. Can you explain this answer?
The number of radial nodes for 3p orbital is __________.
a)
3
b)
4
c)
2
d)
1

|
Amrita Kumar answered |
Number of radial nodes = n-1 – 1
For 3p orbital, n = 3 – 1 – 1 = 1
Number of radial nodes = 3 – 1 – 1 = 1.
For 3p orbital, n = 3 – 1 – 1 = 1
Number of radial nodes = 3 – 1 – 1 = 1.
Values of e/m (charge/mass) in the categories alpha particle (α), electron (e) and protons (p) increase in the order:
- a)α < e < p
- b)p < e < α
- c)e < α < p
- d)α< p < e
Correct answer is option 'D'. Can you explain this answer?
Values of e/m (charge/mass) in the categories alpha particle (α), electron (e) and protons (p) increase in the order:
a)
α < e < p
b)
p < e < α
c)
e < α < p
d)
α< p < e
|
|
Hansa Sharma answered |
E/m values of particle (α), electron (e) and protons (p) increase in the order α< p < e
The spectrum of He is expected to be similar to that[1988]- a)H
- b)Li+
- c)Na
- d)He+
Correct answer is option 'B'. Can you explain this answer?
The spectrum of He is expected to be similar to that[1988]
a)
H
b)
Li+
c)
Na
d)
He+

|
Arindam Khanna answered |
Both He and Li+ contain 2 electrons each therefore their spectrum will be similar.
Isobars are the atoms with- a)same atomic number but different number of neutrons
- b)same mass number but different atomic number
- c)same atomic number but different mass number
- d)same number of neutrons but different mass number
Correct answer is option 'B'. Can you explain this answer?
Isobars are the atoms with
a)
same atomic number but different number of neutrons
b)
same mass number but different atomic number
c)
same atomic number but different mass number
d)
same number of neutrons but different mass number
|
|
Nandini Patel answered |
Isobars are atoms (nuclides) of different chemical elements that have the same number of nucleons. Correspondingly, isobars differ in atomic number (or number of protons) but have the same mass number.
The nature of positive rays depends on?
- a)The nature of discharge tube.
- b)The nature of residual gas.
- c)The nature of electrode.
- d)All of above
Correct answer is option 'B'. Can you explain this answer?
The nature of positive rays depends on?
a)
The nature of discharge tube.
b)
The nature of residual gas.
c)
The nature of electrode.
d)
All of above
|
|
Om Desai answered |
- The nature of positive rays produced in a vacuum discharge tube depends upon the nature of the gas-filled.
- The positive rays consist of positive ions obtained by removing one or more electrons from gas molecules.
Most penetrating radiation of radioactive element is:- a) β — rays
- b) α — rays
- c) x — rays
- d) γ — rays
Correct answer is option 'D'. Can you explain this answer?
Most penetrating radiation of radioactive element is:
a)
β — rays
b)
α — rays
c)
x — rays
d)
γ — rays
|
|
Anjana Sharma answered |
Alpha rays as the least penetrating, followed by beta rays, followed by gamma rays as the most penetrating.
The energy associated with the first orbit in the hydrogen atom is -2.18 x 10−18 J/atom. What is the energy associated with the fifth orbit?
a) -7.72 ×10−20 J/atom
b) -5.72 ×10−20 J/atom
c) -3.72 ×10−20 J/atom
d) -8.72 ×10−20 J/atom
Correct answer is 'D'. Can you explain this answer?
|
|
Geetika Shah answered |
Energy for any n shell = -2.18 x 10−18 )/n'2 (') stands for raise to the power of therefore, E associated with 5 orbit = -2.18 x 10−18/52 = -2.18 × 10-18/25 = -218 × 10-20/25 = -8.72 x 10-20J/atom
The de – Broglie wavelength of an electron is 600 nm. The velocity of the electron having the mass 9.1 X 10-31 Kg is- a)0.0012 x 10+4 m/s
- b)0.0012 x 10+3 m/s
- c)0.0012 x 10+6 m/s
- d)0.0012 x 10+2 m/s
Correct answer is option 'C'. Can you explain this answer?
The de – Broglie wavelength of an electron is 600 nm. The velocity of the electron having the mass 9.1 X 10-31 Kg is
a)
0.0012 x 10+4 m/s
b)
0.0012 x 10+3 m/s
c)
0.0012 x 10+6 m/s
d)
0.0012 x 10+2 m/s
|
|
Arjun Gupta answered |
1nm= 10^-9m.
wavelength= 600nm= 600 ×10-9m
wavelength= h/p.
wavelength= h/mv
v= h/ m wavelength
v=6.625×10^-34/9.1×10^-31×600× 10-9.
v= 6.625 × 10+4/9.1 ×6.
v= 6.625× 10+4/9.1×6.
v= 6.625 ×104/54.6.
v= 0.12 × 104.
v= 0.0012 × 10+6 .
wavelength= 600nm= 600 ×10-9m
wavelength= h/p.
wavelength= h/mv
v= h/ m wavelength
v=6.625×10^-34/9.1×10^-31×600× 10-9.
v= 6.625 × 10+4/9.1 ×6.
v= 6.625× 10+4/9.1×6.
v= 6.625 ×104/54.6.
v= 0.12 × 104.
v= 0.0012 × 10+6 .
Which model describes that there is no change in the energy of electrons as long as they keep revolving in the same energy level and atoms remains stable?- a)Rutherford Model
- b)Bohr’s Model
- c)J.J Thomson Model
- d)None of the above
Correct answer is option 'B'. Can you explain this answer?
Which model describes that there is no change in the energy of electrons as long as they keep revolving in the same energy level and atoms remains stable?
a)
Rutherford Model
b)
Bohr’s Model
c)
J.J Thomson Model
d)
None of the above
|
|
Suresh Reddy answered |
Bohr Model of atom:
- An atom is made up of three particles: Electrons, neutrons and protons.
- The protons and neutrons are located in a small nucleus at the centre of the atom.
- The electrons revolve rapidly around the nucleus at the centre of the atom.
- There is a limit to the number of electrons that each energy level can hold.
- Each energy level is associated with a fixed amount of energy.
- There is no change in the energy of electrons as long as they keep revolving in the same energy level.
Bohr explained the stability through the concept of revolution of electrons in different energy levels.
The change in the energy of an electron occurs when it jumps from lower to higher energy levels. When it gains energy, it excites from lower to higher and vice versa.
Thus energy is not lost and the atom remains stable.
The change in the energy of an electron occurs when it jumps from lower to higher energy levels. When it gains energy, it excites from lower to higher and vice versa.
Thus energy is not lost and the atom remains stable.
The de Broglie wavelength of an electron is 8.7 x 10-11 m. The mass of an electron is 9.1 x 10-31 kg. The velocity of this electron is:- a)0.0837 × 108 m/s
- b)6.9 × 10-5 m/s
- c)8.4 × 103 m/s
- d)1.2 × 10-7 m/s
Correct answer is option 'A'. Can you explain this answer?
The de Broglie wavelength of an electron is 8.7 x 10-11 m. The mass of an electron is 9.1 x 10-31 kg. The velocity of this electron is:
a)
0.0837 × 108 m/s
b)
6.9 × 10-5 m/s
c)
8.4 × 103 m/s
d)
1.2 × 10-7 m/s
|
|
Suresh Reddy answered |
Wavelength = h/p = h/ mv.
v= h/ m.wavelength
v= 6.625 × 10-34/9.1×10-31 × 8.7 × 10-11
v= 6.625 × 108/ 9.1× 8.7
v= 0.08367 ×108.
v= h/ m.wavelength
v= 6.625 × 10-34/9.1×10-31 × 8.7 × 10-11
v= 6.625 × 108/ 9.1× 8.7
v= 0.08367 ×108.
The nature of positive rays depends on?- a) The nature of discharge tube.
- b) The nature of residual gas
- c) All of above.
- d) The nature of electrode
Correct answer is option 'B'. Can you explain this answer?
The nature of positive rays depends on?
a)
The nature of discharge tube.
b)
The nature of residual gas
c)
All of above.
d)
The nature of electrode

|
Abhiram Choudhary answered |
The positive charges in these rays, other than negative cathode rays (which are electrons), depend on the gas that is used because they are cations - atoms with mostly one electron missing and thus one positive charge. So, if you accelerate, argon cations and protons over the same electric potential, the particles in the rays will have the same kinetic energy, but the argon ions will be much slower, as they are much heavier than the protons.
Uncertainty in the position of an electron (mass 9.1 x 10-31 kg) moving with a velocity of 300 ms-1, accurate upto 0.001% will be:
- a)1.92 x 10-2 m
- b)3.84 x 10-2 m
- c)19.2 x 10-2 m
- d)5.76 x 10-2 m
Correct answer is option 'C'. Can you explain this answer?
Uncertainty in the position of an electron (mass 9.1 x 10-31 kg) moving with a velocity of 300 ms-1, accurate upto 0.001% will be:
a)
1.92 x 10-2 m
b)
3.84 x 10-2 m
c)
19.2 x 10-2 m
d)
5.76 x 10-2 m
|
|
Lavanya Menon answered |
Change in position (X),
P= momentum,
P=mv.
X ×P = h/4π.
X× m v = h/4π.
X= h/4πmv.
X=6.625×10-34/4 ×3.14×9.1×10^-31×300.
X=6.625×10-5/342.8.
X=0.0192×10^-5.
X= 19.2× 10-7.
in question it is given to take accurate up to 0.001%.= 1×10-3/100=1 ×10-5.
X= 19.2 ×10-7/10-5.
X=19.2 × 10-2.
P= momentum,
P=mv.
X ×P = h/4π.
X× m v = h/4π.
X= h/4πmv.
X=6.625×10-34/4 ×3.14×9.1×10^-31×300.
X=6.625×10-5/342.8.
X=0.0192×10^-5.
X= 19.2× 10-7.
in question it is given to take accurate up to 0.001%.= 1×10-3/100=1 ×10-5.
X= 19.2 ×10-7/10-5.
X=19.2 × 10-2.
Energy of a mole of radio wave photons with a frequency of 909 kHz is- a)6.02 x 10-28 J
- b)3.62 x 10-4 J
- c)1.00 x 10-4 J
- d)6.02 x 10-31 J
Correct answer is option 'B'. Can you explain this answer?
Energy of a mole of radio wave photons with a frequency of 909 kHz is
a)
6.02 x 10-28 J
b)
3.62 x 10-4 J
c)
1.00 x 10-4 J
d)
6.02 x 10-31 J
|
|
Rajeev Nair answered |
E = N0hv
= 6.02 x 1023 x 6.62 x 10-34 Js x 909 x103 s-1
= 3.62 x 10-4 J
= 3.62 x 10-4 J
The number of radial nodes for 3p orbital is __________.- a)3
- b)4
- c)2
- d)1
Correct answer is option 'D'. Can you explain this answer?
The number of radial nodes for 3p orbital is __________.
a)
3
b)
4
c)
2
d)
1

|
Swara Saha answered |
Number of radial nodes = n-1 – 1
For 3p orbital, n = 3 – 1 – 1 = 1
Number of radial nodes = 3 – 1 – 1 = 1.
For 3p orbital, n = 3 – 1 – 1 = 1
Number of radial nodes = 3 – 1 – 1 = 1.
In the relationship ∆x. ∆p = 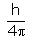 , ∆p is:
, ∆p is:- a)Certainty in momentum
- b)Certainty in position
- c)Uncertainty in momentum
- d)Uncertainty in position
Correct answer is option 'C'. Can you explain this answer?
In the relationship ∆x. ∆p = 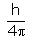 , ∆p is:
, ∆p is:
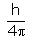 , ∆p is:
, ∆p is:a)
Certainty in momentum
b)
Certainty in position
c)
Uncertainty in momentum
d)
Uncertainty in position
|
|
Hansa Sharma answered |
The uncertainty principle is alternatively expressed in terms of a particle’s momentum and position. The momentum of a particle is equal to the product of its mass times its velocity. Thus, the product of the uncertainties in the momentum and the position of a particle equals h/(4π)
The frequency of radiation emitted when the electron falls from n = 4 to n = 1 in a hydrogen atom will be (Given ionization energy of H=2.18 ×10–18J atom–1and h = 6.625 × 10–34 J s ) [2004]- a)1.54 x 1015 s -1
- b)1.03 x 1015 s -1
- c)3.08 x 1015 s -1
- d)2.00 x 1015 s -1
Correct answer is option 'C'. Can you explain this answer?
The frequency of radiation emitted when the electron falls from n = 4 to n = 1 in a hydrogen atom will be (Given ionization energy of H=2.18 ×10–18J atom–1and h = 6.625 × 10–34 J s ) [2004]
a)
1.54 x 1015 s -1
b)
1.03 x 1015 s -1
c)
3.08 x 1015 s -1
d)
2.00 x 1015 s -1

|
Harshitha Dey answered |
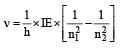
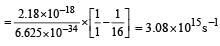
Chapter doubts & questions for Structure of Atom - Topic-wise MCQ Tests for NEET 2025 is part of NEET exam preparation. The chapters have been prepared according to the NEET exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for NEET 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Structure of Atom - Topic-wise MCQ Tests for NEET in English & Hindi are available as part of NEET exam.
Download more important topics, notes, lectures and mock test series for NEET Exam by signing up for free.
Topic-wise MCQ Tests for NEET
9 docs|806 tests
|