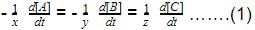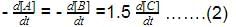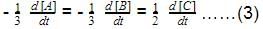All Exams >
NEET >
Chemistry CUET UG Mock Test Series 2026 >
All Questions
All questions of Chemical Kinetics for NEET Exam
Direction (Q. Nos. 14 and 15) This section contains a passage describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer out of the given 4 options (a), (b), (c) and (d)
Passage
The decomposition of NO2 at 400 K proceeds at a of rate of 5.4 x 10 -5 mol L-1 s-1 when [NO2] = 0.01 mol-1
2 NO2(g) → 2NO(g ) + O2(g).
Q. What is the rate law when observed rate is 1.35 x 10-5 mol L-1 s-1 at [NO2] = 0.005 mol L-1?
- a)k[NO2]
- b)k[NO2]0
- c)k[NO2]3
- d)k[NO2]2
Correct answer is option 'D'. Can you explain this answer?
Direction (Q. Nos. 14 and 15) This section contains a passage describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer out of the given 4 options (a), (b), (c) and (d)
Passage
The decomposition of NO2 at 400 K proceeds at a of rate of 5.4 x 10 -5 mol L-1 s-1 when [NO2] = 0.01 mol-1
2 NO2(g) → 2NO(g ) + O2(g).
Q. What is the rate law when observed rate is 1.35 x 10-5 mol L-1 s-1 at [NO2] = 0.005 mol L-1?
a)
k[NO2]
b)
k[NO2]0
c)
k[NO2]3
d)
k[NO2]2
|
|
Geetika Shah answered |
Given unit of rate = mol L-1 s-1
General formula for unit of rate = mol n-1 Ln-1 s -1
On equating,
1-n = 1 ⇒ n = 0
n-1 = -1 ⇒ n = 0
General formula for unit of rate = mol n-1 Ln-1 s -1
On equating,
1-n = 1 ⇒ n = 0
n-1 = -1 ⇒ n = 0
Hence, it is a zero order reaction.
A first order reaction is 50% completed in 20 minutes at 27°C and in 5 min at 47°C. The energy of activation of the reaction is- a)43.85 kJ/mol
- b)55.3 kJ/mol
- c)11.97 kJ/mol
- d)6.65 kJ/mol
Correct answer is option 'B'. Can you explain this answer?
A first order reaction is 50% completed in 20 minutes at 27°C and in 5 min at 47°C. The energy of activation of the reaction is
a)
43.85 kJ/mol
b)
55.3 kJ/mol
c)
11.97 kJ/mol
d)
6.65 kJ/mol
|
|
Tanuja Kapoor answered |
t1/2 = 20 min at 300 K
t’1/2 = 5 min at 320 K
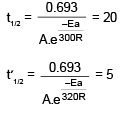
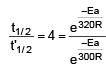
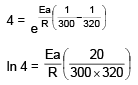
On solving, Ea = 55303.12 J
= 55.3 KJ
t’1/2 = 5 min at 320 K
On solving, Ea = 55303.12 J
= 55.3 KJ
The ratio of the rate constant of a reaction at two temperatures differing by __________0C is called temperature coefficient of reaction.- a)2
- b)10
- c)100
- d)50
Correct answer is option 'B'. Can you explain this answer?
The ratio of the rate constant of a reaction at two temperatures differing by __________0C is called temperature coefficient of reaction.
a)
2
b)
10
c)
100
d)
50

|
Devika Banerjee answered |
The ratio of the rate constant of a reaction at two temperatures differing by 100C is called temperature coefficient of reaction.
The initial rates of reaction for the equation, 2A + B → Products.Products were determined under various initial concentrations of reactants. Thus, rate law is equal to
Thus, rate law is equal to- a)k[A][B]
- b)k[A][B]2
- c) k[A]
- d) k[B]
Correct answer is option 'C'. Can you explain this answer?
The initial rates of reaction for the equation, 2A + B → Products.
Products were determined under various initial concentrations of reactants.
Thus, rate law is equal to
a)
k[A][B]
b)
k[A][B]2
c)
k[A]
d)
k[B]
|
|
Ritu Singh answered |
Let order w.r.t. A = a, order w.r.t. B = b
If 75% of a first order reaction was completed in 32 min, then 50% of the reaction was completed in- a)24 min
- b)4 min
- c)16 min
- d)8 min
Correct answer is option 'C'. Can you explain this answer?
If 75% of a first order reaction was completed in 32 min, then 50% of the reaction was completed in
a)
24 min
b)
4 min
c)
16 min
d)
8 min

|
Avi Chawla answered |
75% completion means 2 half lifes so 50% completion means only one half life.
The time for half life period of a certain reaction A → Products is 1 h. When the initial concentration of the reactant 'A' is 2.0 mol L–1, how much time does it take for its concentration to come from 0.50 to 0.25 mol L–1, if it is a zero order reaction?- a)0.25 h
- b)1 h
- c)4 h
- d)0.5 h
Correct answer is option 'A'. Can you explain this answer?
The time for half life period of a certain reaction A → Products is 1 h. When the initial concentration of the reactant 'A' is 2.0 mol L–1, how much time does it take for its concentration to come from 0.50 to 0.25 mol L–1, if it is a zero order reaction?
a)
0.25 h
b)
1 h
c)
4 h
d)
0.5 h

|
Shashi Kumar answered |
For a zero order reaction,
(T)half = Initial concentration/ 2× reaction constant
from this equation find out k.
also another formula for zero order reaction-
(A)t =(A)o - KT
put the value of k , (A)t=0.25 and (A)o= 0.50
you will get the answer.
(T)half = Initial concentration/ 2× reaction constant
from this equation find out k.
also another formula for zero order reaction-
(A)t =(A)o - KT
put the value of k , (A)t=0.25 and (A)o= 0.50
you will get the answer.
The effect of temperature on reaction rate is given by- a)Arrhenius equation
- b)Kirchoff’s Equation
- c)Clauius Claperyron equation
- d)Gibb’s Helmholtz equation
Correct answer is option 'A'. Can you explain this answer?
The effect of temperature on reaction rate is given by
a)
Arrhenius equation
b)
Kirchoff’s Equation
c)
Clauius Claperyron equation
d)
Gibb’s Helmholtz equation
|
|
Shanaya Choudhary answered |
Effect of Temperature on Reaction Rate - Arrhenius Equation
The Arrhenius equation explains the effect of temperature on the reaction rate. It is a mathematical formula that relates the rate constant of a chemical reaction to the temperature and activation energy of the reaction.
The equation is given by:
k = A * e^(-Ea/RT)
Where,
k - rate constant
A - pre-exponential factor
Ea - activation energy
R - gas constant
T - temperature
Explanation of the Equation:
Pre-Exponential Factor (A): It is a constant that reflects the frequency at which reactant molecules collide with each other. A higher value of A means that more collisions occur, thus increasing the reaction rate.
Activation Energy (Ea): It is the minimum energy required to initiate a chemical reaction. A higher value of Ea means that more energy is required to initiate the reaction, thus decreasing the reaction rate.
Gas Constant (R): It is a constant that relates the energy of a system to its temperature.
Temperature (T): It is the measure of the average kinetic energy of the molecules in a system. As the temperature increases, the kinetic energy of the molecules increases, leading to more collisions and thus increasing the reaction rate.
Application of the Equation:
The Arrhenius equation is widely used in chemical kinetics to predict the effect of temperature on the reaction rate. It is also used to determine the activation energy of a reaction by measuring the rate constant at different temperatures.
Conclusion:
In conclusion, the Arrhenius equation is a useful tool to understand the effect of temperature on the reaction rate. It helps in predicting the rate of a chemical reaction at different temperatures and determining the activation energy of a reaction.
The Arrhenius equation explains the effect of temperature on the reaction rate. It is a mathematical formula that relates the rate constant of a chemical reaction to the temperature and activation energy of the reaction.
The equation is given by:
k = A * e^(-Ea/RT)
Where,
k - rate constant
A - pre-exponential factor
Ea - activation energy
R - gas constant
T - temperature
Explanation of the Equation:
Pre-Exponential Factor (A): It is a constant that reflects the frequency at which reactant molecules collide with each other. A higher value of A means that more collisions occur, thus increasing the reaction rate.
Activation Energy (Ea): It is the minimum energy required to initiate a chemical reaction. A higher value of Ea means that more energy is required to initiate the reaction, thus decreasing the reaction rate.
Gas Constant (R): It is a constant that relates the energy of a system to its temperature.
Temperature (T): It is the measure of the average kinetic energy of the molecules in a system. As the temperature increases, the kinetic energy of the molecules increases, leading to more collisions and thus increasing the reaction rate.
Application of the Equation:
The Arrhenius equation is widely used in chemical kinetics to predict the effect of temperature on the reaction rate. It is also used to determine the activation energy of a reaction by measuring the rate constant at different temperatures.
Conclusion:
In conclusion, the Arrhenius equation is a useful tool to understand the effect of temperature on the reaction rate. It helps in predicting the rate of a chemical reaction at different temperatures and determining the activation energy of a reaction.
The reactions with low activation energy are- a)Slow
- b)Non-spontaneous
- c)Fast
- d)Always spontaneous
Correct answer is option 'C'. Can you explain this answer?
The reactions with low activation energy are
a)
Slow
b)
Non-spontaneous
c)
Fast
d)
Always spontaneous
|
|
Vivek Godara answered |
Low activation energy mean energy reguire for reaction to occur is low so product making is fast
The rate of a chemical reaction doubles for every 10°C rise of temperature. If the temperature is raised by 50°C, the rate of the reaction increases by about- a)64 times
- b)10 times
- c)24 times
- d)32 times
Correct answer is option 'D'. Can you explain this answer?
The rate of a chemical reaction doubles for every 10°C rise of temperature. If the temperature is raised by 50°C, the rate of the reaction increases by about
a)
64 times
b)
10 times
c)
24 times
d)
32 times

|
Mohit Rajpoot answered |
For every 10°C rise of temperature, the rate is doubled. Thus, the temperature coefficient of the reaction = 2
When temperature is increased by 50°, rate becomes
=2(50/10) = 25 times = 32 times
When temperature is increased by 50°, rate becomes
=2(50/10) = 25 times = 32 times
Read the passage given below and answer the following questions:The half-life of a reaction is the time required for the concentration of reactant to decrease by half, i.e.,[A]t = [A]/2For first order reaction,t1/2 = 0.693/kThis means t1/2 is independent of initial concentration. Figure shows that typical variation of concentration of reactant exhibiting first order kinetics. It may be noted that though the major portion of the first order kinetics may be over in a finite time, but the reaction will never cease as the concentration of reactant will be zero only at infinite time.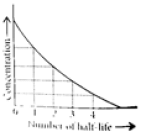 The following questions are multiple choice questions. Choose the most appropriate answer:Q. A first order reaction has a rate constant k = 3.01 x 10-3 /s. How long it will take to decompose half of the reactant?
The following questions are multiple choice questions. Choose the most appropriate answer:Q. A first order reaction has a rate constant k = 3.01 x 10-3 /s. How long it will take to decompose half of the reactant?- a)2.303 s
- b)23.03 s
- c)230.3 s
- d)2303 s
Correct answer is option 'C'. Can you explain this answer?
Read the passage given below and answer the following questions:
The half-life of a reaction is the time required for the concentration of reactant to decrease by half, i.e.,
[A]t = [A]/2
For first order reaction,
t1/2 = 0.693/k
This means t1/2 is independent of initial concentration. Figure shows that typical variation of concentration of reactant exhibiting first order kinetics. It may be noted that though the major portion of the first order kinetics may be over in a finite time, but the reaction will never cease as the concentration of reactant will be zero only at infinite time.

The following questions are multiple choice questions. Choose the most appropriate answer:
Q. A first order reaction has a rate constant k = 3.01 x 10-3 /s. How long it will take to decompose half of the reactant?
a)
2.303 s
b)
23.03 s
c)
230.3 s
d)
2303 s

|
Ambition Institute answered |
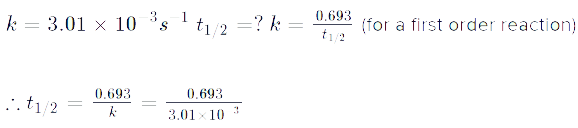
The following mechanism has been proposed for the exothermic catalyzed complex reaction.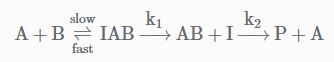 If k1 is much smaller than k2. The most suitable qualitative plot of potential energy (P.E.) versus reaction coordinate for the above reaction.
If k1 is much smaller than k2. The most suitable qualitative plot of potential energy (P.E.) versus reaction coordinate for the above reaction.- a)
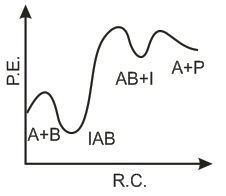
- b)
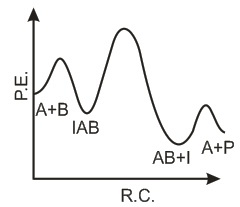
- c)
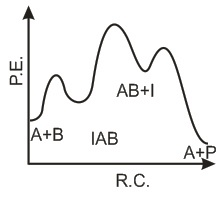
- d)
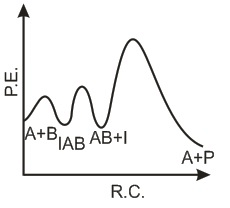
Correct answer is option 'C'. Can you explain this answer?
The following mechanism has been proposed for the exothermic catalyzed complex reaction.
If k1 is much smaller than k2. The most suitable qualitative plot of potential energy (P.E.) versus reaction coordinate for the above reaction.
a)

b)

c)

d)


|
Imk Pathsala answered |
Since
K1 <<< K2 = most Imp. peack will be higher
The rate law for a reaction between the substances A and 8 is given byrate = k[A]n [B]mIf concentration of A is doubled and that of 8 is halved, the new rate as compared to the earlier rate would be - a)
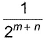
- b)(m + n)
- c)(n - m)
- d)2(n-m)
Correct answer is option 'D'. Can you explain this answer?
The rate law for a reaction between the substances A and 8 is given by
rate = k[A]n [B]m
If concentration of A is doubled and that of 8 is halved, the new rate as compared to the earlier rate would be
a)
b)
(m + n)
c)
(n - m)
d)
2(n-m)

|
Top Rankers answered |
Direction (Q. Nos. 1-13) This section contains multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correctQ. In the following reaction, which has maximum rate w.r.t. rate of disappearance of NH3?4NH3 + 502 → 4NO + 6H2O- a)O2
- b)NO
- c)H2O
- d)Equal
Correct answer is option 'C'. Can you explain this answer?
Direction (Q. Nos. 1-13) This section contains multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct
Q. In the following reaction, which has maximum rate w.r.t. rate of disappearance of NH3?
4NH3 + 502 → 4NO + 6H2O
a)
O2
b)
NO
c)
H2O
d)
Equal

|
Nabanita Basu answered |
Understanding the Reaction
The given reaction is:
4NH3 + 5O2 → 4NO + 6H2O
This reaction involves the disappearance of ammonia (NH3) and the appearance of the products NO and H2O.
Rate of Reaction
The rate of a chemical reaction can be expressed in terms of the rate of disappearance of reactants or the rate of appearance of products.
Stoichiometry of the Reaction
- According to the stoichiometry:
- 4 moles of NH3 produce 4 moles of NO and 6 moles of H2O.
- The coefficients in the balanced equation indicate the relative rates of disappearance and appearance.
Rate of Disappearance
- The rate of disappearance of NH3 is given by:
Rate = - (1/4) * d[NH3]/dt
- The rates for O2, NO, and H2O can be expressed similarly:
- O2: Rate = - (1/5) * d[O2]/dt
- NO: Rate = (1/4) * d[NO]/dt
- H2O: Rate = (1/6) * d[H2O]/dt
Comparison of Rates
To find the maximum rate of disappearance, we can compare the rates derived from the balanced equation:
- NH3: - (1/4) (for every 1 mole of disappearance)
- O2: - (1/5) (for every 1 mole of disappearance)
- NO: (1/4) (for every 1 mole of appearance)
- H2O: (1/6) (for every 1 mole of appearance)
The fractions reveal how many moles of each substance are involved in the reaction. The lower the denominator, the higher the rate of disappearance or appearance.
Conclusion
- Among the reactants and products, H2O has the highest coefficient when considering the rate of disappearance of NH3.
- Therefore, the maximum rate of disappearance is related to H2O's formation.
Thus, the correct answer is option 'C' (H2O).
The given reaction is:
4NH3 + 5O2 → 4NO + 6H2O
This reaction involves the disappearance of ammonia (NH3) and the appearance of the products NO and H2O.
Rate of Reaction
The rate of a chemical reaction can be expressed in terms of the rate of disappearance of reactants or the rate of appearance of products.
Stoichiometry of the Reaction
- According to the stoichiometry:
- 4 moles of NH3 produce 4 moles of NO and 6 moles of H2O.
- The coefficients in the balanced equation indicate the relative rates of disappearance and appearance.
Rate of Disappearance
- The rate of disappearance of NH3 is given by:
Rate = - (1/4) * d[NH3]/dt
- The rates for O2, NO, and H2O can be expressed similarly:
- O2: Rate = - (1/5) * d[O2]/dt
- NO: Rate = (1/4) * d[NO]/dt
- H2O: Rate = (1/6) * d[H2O]/dt
Comparison of Rates
To find the maximum rate of disappearance, we can compare the rates derived from the balanced equation:
- NH3: - (1/4) (for every 1 mole of disappearance)
- O2: - (1/5) (for every 1 mole of disappearance)
- NO: (1/4) (for every 1 mole of appearance)
- H2O: (1/6) (for every 1 mole of appearance)
The fractions reveal how many moles of each substance are involved in the reaction. The lower the denominator, the higher the rate of disappearance or appearance.
Conclusion
- Among the reactants and products, H2O has the highest coefficient when considering the rate of disappearance of NH3.
- Therefore, the maximum rate of disappearance is related to H2O's formation.
Thus, the correct answer is option 'C' (H2O).
Reaction kinetics deals with the study of- a)Rate of reaction
- b)Mechanism of reaction
- c)Factors which affects the rate of reaction
- d)All of the mentioned
Correct answer is option 'D'. Can you explain this answer?
Reaction kinetics deals with the study of
a)
Rate of reaction
b)
Mechanism of reaction
c)
Factors which affects the rate of reaction
d)
All of the mentioned
|
|
Om Desai answered |
Reaction kinetics deals with the study of rate of reaction, their mechanism and the factors which affects the rate of reaction. It specifies all the general characteristics of a chemical reaction.
The rate is independent of the concentration of the reactants in- a)First order
- b)Second order
- c)Third order
- d)Zero order
Correct answer is option 'D'. Can you explain this answer?
The rate is independent of the concentration of the reactants in
a)
First order
b)
Second order
c)
Third order
d)
Zero order
|
|
Naiham Difoesa answered |
Bcz rate is proportional to the zeroth power of the concentration of reactant.
The unit of rate constant for a first order reaction is- a)Mol/L
- b)Mol2 / L2 / S2
- c)S-1
- d)Mol/L/S
Correct answer is option 'C'. Can you explain this answer?
The unit of rate constant for a first order reaction is
a)
Mol/L
b)
Mol2 / L2 / S2
c)
S-1
d)
Mol/L/S
|
|
Nikita Singh answered |
The correct answer is Option C.
Let R be the rate of reaction.
For first order reaction,
R=K[A]1
⇒K=R[A]-1
Whereas, K and [A] are rate constant and initial concentration of reactant respectively.
Therefore,
Unit of rate constant =(mol L-1)1-nsec-1
For first order reaction, n=1
Unit of rate constant = sec-1
Hence the unit of rate constant for first order reaction is sec-1.
For first order reaction,
R=K[A]1
⇒K=R[A]-1
Whereas, K and [A] are rate constant and initial concentration of reactant respectively.
Therefore,
Unit of rate constant =(mol L-1)1-nsec-1
For first order reaction, n=1
Unit of rate constant = sec-1
Hence the unit of rate constant for first order reaction is sec-1.
A Certain Zeroth Order reaction has k = 0.025 Ms-1 for the disappearance of A. What will be the concentration of A after 15s, if the intial concentration is 0.50 M?- a)0.50 M
- b)0.375 M
- c)0.125 M
- d)0.060 M
Correct answer is option 'C'. Can you explain this answer?
A Certain Zeroth Order reaction has k = 0.025 Ms-1 for the disappearance of A. What will be the concentration of A after 15s, if the intial concentration is 0.50 M?
a)
0.50 M
b)
0.375 M
c)
0.125 M
d)
0.060 M

|
Sinjini Tiwari answered |
x (product) formed after 15 s = 0.025 Ms-1 x 15s= 0.375 M Then, reactant left = 0.500 - 0.375= 0.125 M
Direction (Q. Nos. 1-13) This section contains multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correctQ. In the following reaction, which has maximum rate w.r.t. rate of disappearance of NH3?4NH3 + 502 → 4NO + 6H2O- a)O2
- b)NO
- c)H2O
- d)Equal
Correct answer is 'C'. Can you explain this answer?
Direction (Q. Nos. 1-13) This section contains multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct
Q. In the following reaction, which has maximum rate w.r.t. rate of disappearance of NH3?
4NH3 + 502 → 4NO + 6H2O
a)
O2
b)
NO
c)
H2O
d)
Equal
|
|
Manoj Chauhan answered |
By stoichiometry of the reaction

similarly,
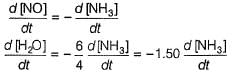
Study of rate of chemical reaction is known as- a)Chemical thermodynamics
- b)Chemical kinetics
- c)Chemical equilibrium
- d)Chemical change
Correct answer is option 'B'. Can you explain this answer?
Study of rate of chemical reaction is known as
a)
Chemical thermodynamics
b)
Chemical kinetics
c)
Chemical equilibrium
d)
Chemical change

|
Sarthak Khanna answered |
Chemical Kinetics deals with rate of a Chemical reactions.
Two reactions with different activation energies have the same rate at room temperature. Which statement correctly describes the rates of these two reactions at the same higher temperature?- a)The reaction with the greater activation energy will be faster
- b)The reaction with the smaller activation energy will be faster
- c)The two reactions will have the same rate at higher temperature also
- d)Temperature range is also required
Correct answer is option 'B'. Can you explain this answer?
Two reactions with different activation energies have the same rate at room temperature. Which statement correctly describes the rates of these two reactions at the same higher temperature?
a)
The reaction with the greater activation energy will be faster
b)
The reaction with the smaller activation energy will be faster
c)
The two reactions will have the same rate at higher temperature also
d)
Temperature range is also required

|
Pranjal Pillai answered |
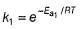

If temperature is increased to T`,
If 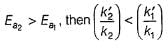
Thus, reaction with smaller activation energy will be faster on increasing temperature.
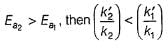
Thus, reaction with smaller activation energy will be faster on increasing temperature.
For the reaction, 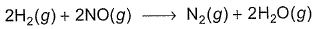
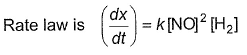 Mechanism is given by
Mechanism is given by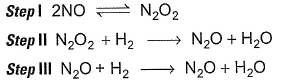 Rate law is true, if
Rate law is true, if - a)step I is the slow step
- b)step II is the slow step
- c)step III is the slow step
- d)step I and step II are the slow steps
Correct answer is option 'B'. Can you explain this answer?
For the reaction, 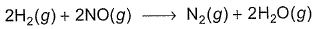
Mechanism is given by
Rate law is true, if
a)
step I is the slow step
b)
step II is the slow step
c)
step III is the slow step
d)
step I and step II are the slow steps

|
Thedot Starry answered |
Yes b is correct because the rate law is written using the slowest step and it matches with second option.
If a reaction proceeds with a uniform rate throughout, the reaction is- a)Third order
- b)Second order
- c)First order
- d)Zero order
Correct answer is option 'D'. Can you explain this answer?
If a reaction proceeds with a uniform rate throughout, the reaction is
a)
Third order
b)
Second order
c)
First order
d)
Zero order

|
Roshni Chavan answered |
Reaction Rate and Order:
The rate of a chemical reaction refers to how quickly reactants are consumed and products are formed. It is determined by the rate equation, which shows how the concentration of reactants influences the rate of the reaction. The order of a reaction refers to the power to which the concentration of a reactant is raised in the rate equation.
Uniform Rate throughout the Reaction:
When a reaction proceeds with a uniform rate throughout, it means that the rate of the reaction remains constant over time. This implies that the concentration of the reactants does not affect the reaction rate. In other words, the reaction is not dependent on the concentration of the reactants.
Zero Order Reaction:
When the reaction rate is independent of the concentration of the reactants, it is referred to as a zero order reaction. In this case, the rate equation is expressed as:
Rate = k
where k is the rate constant. The rate of the reaction is solely determined by the value of the rate constant, and it does not change with changes in reactant concentrations.
Explanation:
In the given question, if the reaction proceeds with a uniform rate throughout, it indicates that the rate of the reaction does not depend on the concentration of the reactants. This is a characteristic of a zero order reaction.
The rate equation for a zero order reaction is Rate = k, where k is the rate constant. It means that the reaction proceeds at a constant rate, regardless of the initial concentrations of the reactants. The rate constant determines the speed of the reaction, and it remains constant throughout the reaction.
Therefore, the correct answer is option 'D' - Zero order.
The rate of a chemical reaction refers to how quickly reactants are consumed and products are formed. It is determined by the rate equation, which shows how the concentration of reactants influences the rate of the reaction. The order of a reaction refers to the power to which the concentration of a reactant is raised in the rate equation.
Uniform Rate throughout the Reaction:
When a reaction proceeds with a uniform rate throughout, it means that the rate of the reaction remains constant over time. This implies that the concentration of the reactants does not affect the reaction rate. In other words, the reaction is not dependent on the concentration of the reactants.
Zero Order Reaction:
When the reaction rate is independent of the concentration of the reactants, it is referred to as a zero order reaction. In this case, the rate equation is expressed as:
Rate = k
where k is the rate constant. The rate of the reaction is solely determined by the value of the rate constant, and it does not change with changes in reactant concentrations.
Explanation:
In the given question, if the reaction proceeds with a uniform rate throughout, it indicates that the rate of the reaction does not depend on the concentration of the reactants. This is a characteristic of a zero order reaction.
The rate equation for a zero order reaction is Rate = k, where k is the rate constant. It means that the reaction proceeds at a constant rate, regardless of the initial concentrations of the reactants. The rate constant determines the speed of the reaction, and it remains constant throughout the reaction.
Therefore, the correct answer is option 'D' - Zero order.
Which among the following is an example of first order reaction?- a)Acid catalysed hydrolysis of ethyl acetate
- b)Formation and dissociation of ozone
- c)Inversion of cane sugar
- d)Decomposition of nitrogen pentoxide
Correct answer is option 'D'. Can you explain this answer?
Which among the following is an example of first order reaction?
a)
Acid catalysed hydrolysis of ethyl acetate
b)
Formation and dissociation of ozone
c)
Inversion of cane sugar
d)
Decomposition of nitrogen pentoxide

|
Pooja Pillai answered |
Decomposition of N2O5 is 1st order reaction.
Decomposition of H2O2 was studied by titration against KMnO4 solution. It was found that 0.4 mol of H2O2 was reduced to 0.2 mol in 20 min and to 0.1 mol in 40 min and to 0.05 mol after 1 hr, the order of reaction must be- a)3
- b)1
- c)0
- d)2
Correct answer is option 'B'. Can you explain this answer?
Decomposition of H2O2 was studied by titration against KMnO4 solution. It was found that 0.4 mol of H2O2 was reduced to 0.2 mol in 20 min and to 0.1 mol in 40 min and to 0.05 mol after 1 hr, the order of reaction must be
a)
3
b)
1
c)
0
d)
2

|
Om Kumar answered |
Decomposition of nH2O2 is 1st order.
Which among the following statement is not true for rate constant of a reaction?- a)Rate constant depend upon the concentration of the reactants
- b)Unit of rate constant depend upon the order of reaction
- c)Rate constant has a definite value at a particular temperature
- d)Rate constant changes with temperature
Correct answer is option 'A'. Can you explain this answer?
Which among the following statement is not true for rate constant of a reaction?
a)
Rate constant depend upon the concentration of the reactants
b)
Unit of rate constant depend upon the order of reaction
c)
Rate constant has a definite value at a particular temperature
d)
Rate constant changes with temperature

|
Anjana Sen answered |
Rate constant is independent of concentration of reactant.
Molecularity of a reaction
- a)Cannot be less than 2
- b)Can be zero
- c)Is always a natural number
- d)Can have a fractional values
Correct answer is option 'C'. Can you explain this answer?
Molecularity of a reaction
a)
Cannot be less than 2
b)
Can be zero
c)
Is always a natural number
d)
Can have a fractional values

|
Nandini Nair answered |
Molecularity is defined as the number of molecules, atoms, or radicals that must collide simultaneously in order for the reaction to take place. It is always a natural number and cannot be negative.
A first order reaction is 50% completed in 1.26 × 1014 s. How much time would it take for 100% completion?- a)1.26 × 1015 s
- b)2.52 × 1014 s
- c)2.52 × 1028 s
- d) infinite
Correct answer is option 'D'. Can you explain this answer?
A first order reaction is 50% completed in 1.26 × 1014 s. How much time would it take for 100% completion?
a)
1.26 × 1015 s
b)
2.52 × 1014 s
c)
2.52 × 1028 s
d)
infinite
|
|
Neha Chauhan answered |
The time taken for half the reaction to complete, i.e., the time in which the concentration of a reactant is reduced to half of its original value is called half-life period of the reaction. But it is impossible to perform 100% of the reaction. Whole of the substance never reacts because in every half-life, 50% of the substance reacts. Hence, time taken for 100% completion of a reaction is infinite.
For the reaction,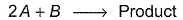 The half-life period was independent of concentration of B. On doubling the concentration A, rate increases two times. Thus, unit of rate constant for this reaction is
The half-life period was independent of concentration of B. On doubling the concentration A, rate increases two times. Thus, unit of rate constant for this reaction is- a)L mol-1s-1
- b)mol L-1s-1
- c)unitless
- d)S-1
Correct answer is option 'A'. Can you explain this answer?
For the reaction,
The half-life period was independent of concentration of B. On doubling the concentration A, rate increases two times. Thus, unit of rate constant for this reaction is
a)
L mol-1s-1
b)
mol L-1s-1
c)
unitless
d)
S-1
|
|
Shanaya Choudhary answered |
I apologize, but your question about the reaction is incomplete. Could you provide more information or detail about the reaction you are referring to?
For a reaction A + B → C + D if the concentration of A is doubled without altering the concentration of B, the rate gets doubled. If the concentration of B is increased by nine times without altering the concentration of A, the rate gets tripled. The order of the reaction is - a)2
- b)1
- c)3/2
- d)4/3
Correct answer is option 'C'. Can you explain this answer?
For a reaction A + B → C + D if the concentration of A is doubled without altering the concentration of B, the rate gets doubled. If the concentration of B is increased by nine times without altering the concentration of A, the rate gets tripled. The order of the reaction is
a)
2
b)
1
c)
3/2
d)
4/3
|
|
Priyanka Sharma answered |
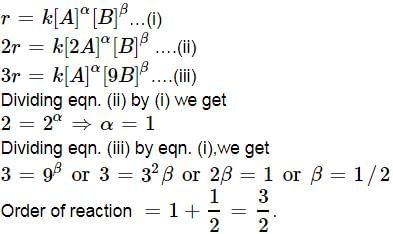
The activation energies of two reactions are given as Ea1= 40 J and Ea2= 80 J, then the relation between their rate constants can be written as:- a)k1 > k2
- b)k1 < k2
- c)k1 = k2
- d)k1 + k2 = 0
Correct answer is option 'A'. Can you explain this answer?
The activation energies of two reactions are given as Ea1= 40 J and Ea2= 80 J, then the relation between their rate constants can be written as:
a)
k1 > k2
b)
k1 < k2
c)
k1 = k2
d)
k1 + k2 = 0
|
|
Vijay Bansal answered |
As the value of activation energy, Ea increases, the value of rate constant, k decreases.
So, k1 > k2 since E1 < E2
Read the passage given below and answer the following questions:The rate of the reaction is proportional to the concentration of the reactant. Hydrogenation of ethene results in the formation of ethane. The rate constant, k for the reaction was found to be 2.5 × 10–15s–1. The concentration of the reactant reduces to one-third of the initial concentration in 5 minutes. The following questions are multiple choice questions. Choose the most appropriate answer:Q. The rate constant of the reaction after 5 minutes is:- a)0.4290 min–1
- b)0.1297 min–1
- c)0.2197 min–1
- d)0.6591 min–1
Correct answer is option 'C'. Can you explain this answer?
Read the passage given below and answer the following questions:
The rate of the reaction is proportional to the concentration of the reactant. Hydrogenation of ethene results in the formation of ethane. The rate constant, k for the reaction was found to be 2.5 × 10–15s–1. The concentration of the reactant reduces to one-third of the initial concentration in 5 minutes. The following questions are multiple choice questions. Choose the most appropriate answer:
Q. The rate constant of the reaction after 5 minutes is:
a)
0.4290 min–1
b)
0.1297 min–1
c)
0.2197 min–1
d)
0.6591 min–1
|
|
Om Desai answered |
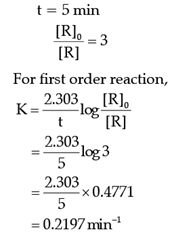
For the first order reaction,A → ProductQ. The concentration of A changes from 0.1 M to 0.025 M in 40 min. The rate of the reaction when the concentration of A is 0.01 M is[AIEEE 2012]- a)1.73 x 10-6 Mmin-1
- b)3.47 x 10-4 M min-1
- c)3.47 x 10-5 M min-1
- d)1.73 x 10-6 M min-1
Correct answer is option 'B'. Can you explain this answer?
For the first order reaction,
A → Product
Q. The concentration of A changes from 0.1 M to 0.025 M in 40 min. The rate of the reaction when the concentration of A is 0.01 M is
[AIEEE 2012]
a)
1.73 x 10-6 Mmin-1
b)
3.47 x 10-4 M min-1
c)
3.47 x 10-5 M min-1
d)
1.73 x 10-6 M min-1

|
Sanjana Bajaj answered |
A first-order reaction is a type of chemical reaction where the rate of reaction is directly proportional to the concentration of one reactant. The rate equation for a first-order reaction can be written as follows:
rate = k[A]
Where:
- rate is the rate of reaction
- k is the rate constant
- [A] is the concentration of reactant A
This means that as the concentration of reactant A decreases, the rate of reaction also decreases. The half-life of a first-order reaction is constant, meaning that it takes the same amount of time for the concentration of reactant A to decrease by half, regardless of the initial concentration.
rate = k[A]
Where:
- rate is the rate of reaction
- k is the rate constant
- [A] is the concentration of reactant A
This means that as the concentration of reactant A decreases, the rate of reaction also decreases. The half-life of a first-order reaction is constant, meaning that it takes the same amount of time for the concentration of reactant A to decrease by half, regardless of the initial concentration.
In a zero-order reaction for every 10° rise of temperature, the rate is doubled. If the temperature is increased from 10°C to 100°C, the rate of the reaction will become - a)64 times
- b)128 times
- c)256 times
- d)512 times
Correct answer is option 'D'. Can you explain this answer?
In a zero-order reaction for every 10° rise of temperature, the rate is doubled. If the temperature is increased from 10°C to 100°C, the rate of the reaction will become
a)
64 times
b)
128 times
c)
256 times
d)
512 times
|
|
Ayaan Madhukar answered |
For rise in temperature, n=1
Therefore rate = 2^n = 2^1 =2
When temperature is increased from 10 degree c to 100 degree c so, change in temperature = 100 -10 = 90 degree c , therefore n = 9 , therefore rate = 2^9 = 512 times...ans...
$$Hope it's help...$$
Therefore rate = 2^n = 2^1 =2
When temperature is increased from 10 degree c to 100 degree c so, change in temperature = 100 -10 = 90 degree c , therefore n = 9 , therefore rate = 2^9 = 512 times...ans...
$$Hope it's help...$$
The rate equation for the reaction,2A + B → Cis found to be, rate = k[A] [B]Q. The correct statement in relation to this reaction is that the- a)unit of k must be s-1
- b)t1/2 is constant
- c)rate of formation of C is half of the rate of disappearance of A
- d)value of k is independent of the initial concentration of A and 8
Correct answer is option 'C'. Can you explain this answer?
The rate equation for the reaction,
2A + B → C
is found to be, rate = k[A] [B]
Q. The correct statement in relation to this reaction is that the
a)
unit of k must be s-1
b)
t1/2 is constant
c)
rate of formation of C is half of the rate of disappearance of A
d)
value of k is independent of the initial concentration of A and 8

|
Amar Jain answered |
Rate = k (A)[B]
The given reaction is first order in A and first order is B.
Thus, total order = 2
Thus, total order = 2
(a) Unit of k = cone 1 - n time -1 = conc-1 time-1 Thus, (a) is false.
(b)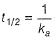 of second-order reaction, thus (b) is false.
of second-order reaction, thus (b) is false.
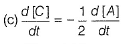
(b)
Thus, (c) is correct.
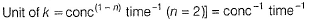
Thus, value of k is dependent on the concentration of A and B. Thus, (d) is false.
Thus, value of k is dependent on the concentration of A and B. Thus, (d) is false.
Chapter doubts & questions for Chemical Kinetics - Chemistry CUET UG Mock Test Series 2026 2025 is part of NEET exam preparation. The chapters have been prepared according to the NEET exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for NEET 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Chemical Kinetics - Chemistry CUET UG Mock Test Series 2026 in English & Hindi are available as part of NEET exam.
Download more important topics, notes, lectures and mock test series for NEET Exam by signing up for free.
Chemistry CUET UG Mock Test Series 2026
3 docs|163 tests
|