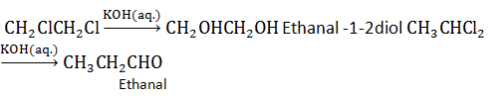All Exams >
ACT >
Science for ACT >
All Questions
All questions of Haloalkanes and Haloarenes for ACT Exam
C5H11Br is least soluble in:- a)Ether
- b)Alcohol
- c)Water
- d)None of these
Correct answer is 'C'. Can you explain this answer?
C5H11Br is least soluble in:
a)
Ether
b)
Alcohol
c)
Water
d)
None of these
|
|
Rajeev Saxena answered |
C5H11Br is only very slightly soluble in water. It is more soluble in organic solvents.
Which of the following is not true about optical isomers?- a)They rotate the plane polarized light.
- b)They are superimposable on their mirror image.
- c)They posses at least one chiral carbon atom.
- d)They are nonsuperimposable on their mirror image.
Correct answer is option 'B'. Can you explain this answer?
Which of the following is not true about optical isomers?
a)
They rotate the plane polarized light.
b)
They are superimposable on their mirror image.
c)
They posses at least one chiral carbon atom.
d)
They are nonsuperimposable on their mirror image.
|
|
Nisha Pillai answered |
Explanation:
Optical isomers are stereoisomers that exist in two mirror-image forms that are non-superimposable on each other. They are also known as enantiomers.
a) They rotate the plane polarized light: Optical isomers have the property of rotating the plane of polarized light in opposite directions. One isomer rotates the plane of polarized light in the clockwise direction and the other in the counterclockwise direction.
b) They are superimposable on their mirror image: This statement is false. Optical isomers are non-superimposable mirror images of each other. If we try to superimpose them, they will not match perfectly.
c) They possess at least one chiral carbon atom: Optical isomers possess chiral carbon atoms. Chiral carbon atoms are those carbon atoms that are attached to four different groups or atoms.
d) They are nonsuperimposable on their mirror image: As mentioned earlier, optical isomers are non-superimposable mirror images of each other. They have the same physical and chemical properties but differ in their biological activity, as they interact differently with other chiral molecules in living organisms.
Conclusion: Optical isomers are important in fields like medicinal chemistry, where knowing the activity of each isomer can help in designing drugs that are more effective and have fewer side effects. Therefore, it is crucial to understand the properties of optical isomers.
Optical isomers are stereoisomers that exist in two mirror-image forms that are non-superimposable on each other. They are also known as enantiomers.
a) They rotate the plane polarized light: Optical isomers have the property of rotating the plane of polarized light in opposite directions. One isomer rotates the plane of polarized light in the clockwise direction and the other in the counterclockwise direction.
b) They are superimposable on their mirror image: This statement is false. Optical isomers are non-superimposable mirror images of each other. If we try to superimpose them, they will not match perfectly.
c) They possess at least one chiral carbon atom: Optical isomers possess chiral carbon atoms. Chiral carbon atoms are those carbon atoms that are attached to four different groups or atoms.
d) They are nonsuperimposable on their mirror image: As mentioned earlier, optical isomers are non-superimposable mirror images of each other. They have the same physical and chemical properties but differ in their biological activity, as they interact differently with other chiral molecules in living organisms.
Conclusion: Optical isomers are important in fields like medicinal chemistry, where knowing the activity of each isomer can help in designing drugs that are more effective and have fewer side effects. Therefore, it is crucial to understand the properties of optical isomers.
Which of the following is a tertiary halogenoalkanes?- a)2-Bromopentane
- b)2-Bromo 3-methylpentane
- c)Bromopentane
- d)2-Bromo 2-methylpentane
Correct answer is option 'D'. Can you explain this answer?
Which of the following is a tertiary halogenoalkanes?
a)
2-Bromopentane
b)
2-Bromo 3-methylpentane
c)
Bromopentane
d)
2-Bromo 2-methylpentane
|
|
Anaya Patel answered |
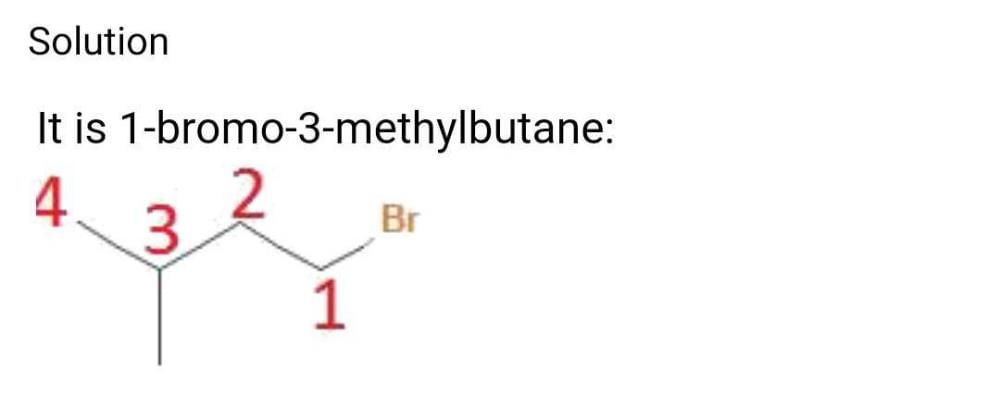
√Br CH3 -( CH )- CH2 - CH2 - CH3 √CH3 The carbon with which the Br is bonded is bonded with another 3 carbon atoms. So haloalkane is 3.
Which of the following compound does have chiral carbon atom?- a)(CH3)2CHCH2CH2Br
- b)CH3CH2CH2CH (Br)CH3
- c)CH3CH2CH(Br) CH2CH3
- d)CH3CH2CH2 CH2Br
Correct answer is 'B'. Can you explain this answer?
Which of the following compound does have chiral carbon atom?
a)
(CH3)2CHCH2CH2Br
b)
CH3CH2CH2CH (Br)CH3
c)
CH3CH2CH(Br) CH2CH3
d)
CH3CH2CH2 CH2Br
|
|
Geetika Shah answered |
The correct answer is Option B.
The chiral carbon atom is one in which there are an unequal number of carbon atoms, this happens only in option (B) as it forms a non superimposable mirror image.
Which of the following is intermediate in Sandmeyer’s reaction?- a)Diazonium salt
- b)Hydronium ion
- c)Ammonium salt
- d)Nitronium ion
Correct answer is option 'A'. Can you explain this answer?
Which of the following is intermediate in Sandmeyer’s reaction?
a)
Diazonium salt
b)
Hydronium ion
c)
Ammonium salt
d)
Nitronium ion
|
|
Rajeev Saxena answered |
Diazonium salt is intermediate in Sandmeyer’s reaction.
Paul Muller of Geigy Pharmaceuticals in Switzerland discovered the effectiveness of DDT as:- a)Insecticides
- b)Fertilizers
- c)Anaesthetics
- d)Antiseptics
Correct answer is option 'A'. Can you explain this answer?
Paul Muller of Geigy Pharmaceuticals in Switzerland discovered the effectiveness of DDT as:
a)
Insecticides
b)
Fertilizers
c)
Anaesthetics
d)
Antiseptics
|
|
Mira Joshi answered |
The correct answer is Option A.
Paul Hermann Muller, Swiss chemist who received the Nobel Prize for Physiology or Medicine in 1948 for discovering the potent toxic effects on insects of DDT. With its chemical derivatives, DDT became the most widely used insecticide for more than 20 years and was a major factor in increased world food production and the suppression of insect-borne diseases.
Paul Hermann Muller, Swiss chemist who received the Nobel Prize for Physiology or Medicine in 1948 for discovering the potent toxic effects on insects of DDT. With its chemical derivatives, DDT became the most widely used insecticide for more than 20 years and was a major factor in increased world food production and the suppression of insect-borne diseases.
The chloro compound which can be used as fire extinguisher is:- a)CH3Cl
- b)COCl2
- c)CCl4
- d)CHCl3
Correct answer is option 'C'. Can you explain this answer?
The chloro compound which can be used as fire extinguisher is:
a)
CH3Cl
b)
COCl2
c)
CCl4
d)
CHCl3
|
|
Om Desai answered |
The correct answer is Option C.
Its use in the home as a spot remover should be avoided because of its poisonous nature.
Because it's vapours (incombustible vapours) are denser than air which leads to a smoother flame.
Because it's vapours (incombustible vapours) are denser than air which leads to a smoother flame.
Which one of the following compounds has zero dipole moment?- a)CCl4
- b)CH3Cl
- c)CHI3
- d)CHCl3
Correct answer is option 'A'. Can you explain this answer?
Which one of the following compounds has zero dipole moment?
a)
CCl4
b)
CH3Cl
c)
CHI3
d)
CHCl3
|
|
Abhijeet Sharma answered |
Explanation:
A dipole moment is the measure of the separation of positive and negative charges in a molecule. It is a vector quantity and has both magnitude and direction. When the molecule has a polar covalent bond, it has a dipole moment. If the bond dipoles cancel out each other, then the molecule is nonpolar, and its dipole moment is zero.
The given compounds are:
a) CCl4
b) CH3Cl
c) CHI3
d) CHCl3
Now, let's analyze each compound based on its molecular geometry and bond polarity to determine its dipole moment.
a) CCl4:
CCl4 has a tetrahedral molecular geometry with four C-Cl bonds arranged symmetrically around the central carbon atom. The C-Cl bond is polar covalent, with chlorine being more electronegative than carbon. However, due to the symmetry of the molecule, the bond dipoles cancel out each other, resulting in a net dipole moment of zero. Therefore, CCl4 has zero dipole moment.
b) CH3Cl:
CH3Cl has a trigonal pyramidal molecular geometry with three C-H bonds and one C-Cl bond. The C-Cl bond is polar covalent, with chlorine being more electronegative than carbon. The three C-H bonds are also polar covalent, with carbon being more electronegative than hydrogen. However, due to the asymmetrical arrangement of the bonds, the bond dipoles do not cancel out each other, resulting in a net dipole moment. Therefore, CH3Cl has a dipole moment.
c) CHI3:
CHI3 has a tetrahedral molecular geometry with three I-H bonds and one I-C bond. The I-C bond is polar covalent, with iodine being more electronegative than carbon. The three I-H bonds are also polar covalent, with iodine being more electronegative than hydrogen. However, due to the symmetry of the molecule, the bond dipoles cancel out each other, resulting in a net dipole moment of zero. Therefore, CHI3 has zero dipole moment.
d) CHCl3:
CHCl3 has a tetrahedral molecular geometry with three C-H bonds and one C-Cl bond. The C-Cl bond is polar covalent, with chlorine being more electronegative than carbon. The three C-H bonds are also polar covalent, with carbon being more electronegative than hydrogen. However, due to the asymmetrical arrangement of the bonds, the bond dipoles do not cancel out each other, resulting in a net dipole moment. Therefore, CHCl3 has a dipole moment.
Conclusion:
Among the given compounds, CCl4 has zero dipole moment because the bond dipoles cancel out each other due to the symmetrical arrangement of the bonds around the central atom.
A dipole moment is the measure of the separation of positive and negative charges in a molecule. It is a vector quantity and has both magnitude and direction. When the molecule has a polar covalent bond, it has a dipole moment. If the bond dipoles cancel out each other, then the molecule is nonpolar, and its dipole moment is zero.
The given compounds are:
a) CCl4
b) CH3Cl
c) CHI3
d) CHCl3
Now, let's analyze each compound based on its molecular geometry and bond polarity to determine its dipole moment.
a) CCl4:
CCl4 has a tetrahedral molecular geometry with four C-Cl bonds arranged symmetrically around the central carbon atom. The C-Cl bond is polar covalent, with chlorine being more electronegative than carbon. However, due to the symmetry of the molecule, the bond dipoles cancel out each other, resulting in a net dipole moment of zero. Therefore, CCl4 has zero dipole moment.
b) CH3Cl:
CH3Cl has a trigonal pyramidal molecular geometry with three C-H bonds and one C-Cl bond. The C-Cl bond is polar covalent, with chlorine being more electronegative than carbon. The three C-H bonds are also polar covalent, with carbon being more electronegative than hydrogen. However, due to the asymmetrical arrangement of the bonds, the bond dipoles do not cancel out each other, resulting in a net dipole moment. Therefore, CH3Cl has a dipole moment.
c) CHI3:
CHI3 has a tetrahedral molecular geometry with three I-H bonds and one I-C bond. The I-C bond is polar covalent, with iodine being more electronegative than carbon. The three I-H bonds are also polar covalent, with iodine being more electronegative than hydrogen. However, due to the symmetry of the molecule, the bond dipoles cancel out each other, resulting in a net dipole moment of zero. Therefore, CHI3 has zero dipole moment.
d) CHCl3:
CHCl3 has a tetrahedral molecular geometry with three C-H bonds and one C-Cl bond. The C-Cl bond is polar covalent, with chlorine being more electronegative than carbon. The three C-H bonds are also polar covalent, with carbon being more electronegative than hydrogen. However, due to the asymmetrical arrangement of the bonds, the bond dipoles do not cancel out each other, resulting in a net dipole moment. Therefore, CHCl3 has a dipole moment.
Conclusion:
Among the given compounds, CCl4 has zero dipole moment because the bond dipoles cancel out each other due to the symmetrical arrangement of the bonds around the central atom.
The inversion of configuration of optically active alkyl halides occurs in- a)SN1 nucleophilic substitution reaction
- b)SN2 electrophilic substitution reaction
- c)SN2 nucleophilic substitution reaction
- d)SN1 electrophilic substitution reaction
Correct answer is option 'C'. Can you explain this answer?
The inversion of configuration of optically active alkyl halides occurs in
a)
SN1 nucleophilic substitution reaction
b)
SN2 electrophilic substitution reaction
c)
SN2 nucleophilic substitution reaction
d)
SN1 electrophilic substitution reaction
|
|
Suresh Iyer answered |
The correct answer is option C
Nucleophilic substitution reaction on an optically active alkyl halide gives a mixture of enantiomers.
Because the reaction occurs by SN1 mechanism.
SN1 mechanism involves racemisation as the nucleophile can attack from either side. In SN2 reaction, inversion of configuration is involved as the nucleophile can attack from the opposite side.
Nucleophilic substitution reaction on an optically active alkyl halide gives a mixture of enantiomers.
Because the reaction occurs by SN1 mechanism.
SN1 mechanism involves racemisation as the nucleophile can attack from either side. In SN2 reaction, inversion of configuration is involved as the nucleophile can attack from the opposite side.
Which one of the following is likely to give a precipitate with AgNO3 solution?- a)CHCl3
- b)(CH3)3CCl
- c)CH2=CH-Cl
- d)CCl4
Correct answer is option 'B'. Can you explain this answer?
Which one of the following is likely to give a precipitate with AgNO3 solution?
a)
CHCl3
b)
(CH3)3CCl
c)
CH2=CH-Cl
d)
CCl4
|
|
Gargi Ahuja answered |
Precipitation Reaction
A precipitation reaction is a chemical reaction that produces a precipitate, which is an insoluble solid that forms from the reaction of two soluble compounds.
AgNO3 Solution
AgNO3 is a soluble salt that dissociates to give Ag+ and NO3- ions in an aqueous solution.
Likely Precipitate
A compound is likely to give a precipitate with AgNO3 solution if it contains Cl- ions, as AgCl is insoluble in water and will form a white precipitate.
Analysis of Options
a) CHCl3
CHCl3 does not contain Cl- ions and will not give a precipitate with AgNO3 solution.
b) (CH3)3CCl
(CH3)3CCl contains Cl- ions and will give a precipitate with AgNO3 solution.
c) CH2=CH-Cl
CH2=CH-Cl does not contain Cl- ions and will not give a precipitate with AgNO3 solution.
d) CCl4
CCl4 does not contain Cl- ions and will not give a precipitate with AgNO3 solution.
Conclusion
Out of the given options, only (CH3)3CCl contains Cl- ions and will give a precipitate with AgNO3 solution, making option 'B' the correct answer.
A precipitation reaction is a chemical reaction that produces a precipitate, which is an insoluble solid that forms from the reaction of two soluble compounds.
AgNO3 Solution
AgNO3 is a soluble salt that dissociates to give Ag+ and NO3- ions in an aqueous solution.
Likely Precipitate
A compound is likely to give a precipitate with AgNO3 solution if it contains Cl- ions, as AgCl is insoluble in water and will form a white precipitate.
Analysis of Options
a) CHCl3
CHCl3 does not contain Cl- ions and will not give a precipitate with AgNO3 solution.
b) (CH3)3CCl
(CH3)3CCl contains Cl- ions and will give a precipitate with AgNO3 solution.
c) CH2=CH-Cl
CH2=CH-Cl does not contain Cl- ions and will not give a precipitate with AgNO3 solution.
d) CCl4
CCl4 does not contain Cl- ions and will not give a precipitate with AgNO3 solution.
Conclusion
Out of the given options, only (CH3)3CCl contains Cl- ions and will give a precipitate with AgNO3 solution, making option 'B' the correct answer.
Which of the following is major product of following reaction?
CH3CH = CH2 + HBr 
- a)2-Bromo propene
- b)1-Bromo propane
- c)1-Bromo propene
- d)2-Bromo propane
Correct answer is option 'D'. Can you explain this answer?
Which of the following is major product of following reaction?
CH3CH = CH2 + HBr
CH3CH = CH2 + HBr

a)
2-Bromo propene
b)
1-Bromo propane
c)
1-Bromo propene
d)
2-Bromo propane
|
|
Geetika Shah answered |
The correct answer is Option D.
As HBr in the presence of a peroxide follows antimarkonikoff rule where the negative group should attach to the carbon having less no of hydrogen atoms.

As HBr in the presence of a peroxide follows antimarkonikoff rule where the negative group should attach to the carbon having less no of hydrogen atoms.
The antiseptic properties of iodoform is due to which of the following:- a)Liberation of iodine
- b)Smell of iodoform
- c)Colour of iodoform
- d)None of the above
Correct answer is option 'A'. Can you explain this answer?
The antiseptic properties of iodoform is due to which of the following:
a)
Liberation of iodine
b)
Smell of iodoform
c)
Colour of iodoform
d)
None of the above
|
|
Alok Verma answered |
The antiseptic properties of iodoform is due to liberation of iodine.
Decreasing order of reactivity of alkyl halide is:- a)RI > RCl > RBr
- b)RI > RBr > RCl
- c)RBr > RCl > RI
- d)RCl > RBr > RI
Correct answer is option 'B'. Can you explain this answer?
Decreasing order of reactivity of alkyl halide is:
a)
RI > RCl > RBr
b)
RI > RBr > RCl
c)
RBr > RCl > RI
d)
RCl > RBr > RI
|
|
Nandini Iyer answered |
On going down the group size of the halides increases and hence stability of the compound decreases as bond length is going to increase resulting in easy break down of the bond and hence reactivity increases.
Thus the correct order of the reactivity is:
R-I >R-Br> R-Cl> R-F
Which of the following compound will not give addition reaction with bromine?- a)Propene
- b)1-Butene
- c)Iso butane
- d)Iso butene
Correct answer is option 'C'. Can you explain this answer?
Which of the following compound will not give addition reaction with bromine?
a)
Propene
b)
1-Butene
c)
Iso butane
d)
Iso butene
|
|
Om Desai answered |
The correct answer is Option C.
For Addition reaction π bond is required .Iso butane has no pi Bond .
Reactivity of alkyl halides towards SN1 nucleophilic substitution reaction is:- a)3° > 2° > 1°
- b)3° < 2° < 1°
- c)2° < 1° < 3°
- d)3° < 1° < 2°
Correct answer is option 'A'. Can you explain this answer?
Reactivity of alkyl halides towards SN1 nucleophilic substitution reaction is:
a)
3° > 2° > 1°
b)
3° < 2° < 1°
c)
2° < 1° < 3°
d)
3° < 1° < 2°
|
|
Swati Verma answered |
The correct answer is option A
Reactivity of alkyl halides towards SN1 nucleophilic substitution reaction is 3o > 2o > 1o because in SN1 nucleophilic reaction, the first and the slow step is the formation of a carbocation. Tertiary carbocation is more stable than a secondary carbocation which is more stable than a primary carbocation. Greater the stability of the carbocation, greater will be the ease of formation of carbocation, and hence faster will be the rate of the reaction.
Reactivity of alkyl halides towards SN1 nucleophilic substitution reaction is 3o > 2o > 1o because in SN1 nucleophilic reaction, the first and the slow step is the formation of a carbocation. Tertiary carbocation is more stable than a secondary carbocation which is more stable than a primary carbocation. Greater the stability of the carbocation, greater will be the ease of formation of carbocation, and hence faster will be the rate of the reaction.
. 
This reaction is known as:- a)Wurtz reaction
- b)Frankland reaction
- c)Fittig reaction
- d)Finkelstein reaction
Correct answer is option 'D'. Can you explain this answer?
. 
This reaction is known as:
This reaction is known as:
a)
Wurtz reaction
b)
Frankland reaction
c)
Fittig reaction
d)
Finkelstein reaction
|
|
Hansa Sharma answered |
The correct answer is option D
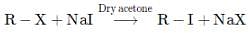
where, X=Cl or Br.
This reaction is known as the Finkelstein reaction. Finkelstein's reaction is used to prepare alkyl iodides starting from alkyl chlorides and alkyl bromides.
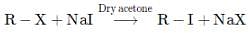
where, X=Cl or Br.
This reaction is known as the Finkelstein reaction. Finkelstein's reaction is used to prepare alkyl iodides starting from alkyl chlorides and alkyl bromides.
Which of the following is formed in reaction mechanism of halogenation of alkanes in presence of UV light?- a)Carbocation
- b)Carbanion
- c)Diazonium ion
- d)Free radical
Correct answer is option 'D'. Can you explain this answer?
Which of the following is formed in reaction mechanism of halogenation of alkanes in presence of UV light?
a)
Carbocation
b)
Carbanion
c)
Diazonium ion
d)
Free radical

|
Niloni Soni answered |
In presence of uv light free radical mechanism takes place that is homolytic cleavage of covalent bond takes place. Carbocation and carbanion and diazonium ion forms when heterolytic cleavage of bond takes place.
Racemic mixture is obtained due to the halogenation of- a)n-pentane
- b)isopentane
- c)neopentane
- d)Both (a) and (b)
Correct answer is 'D'. Can you explain this answer?
Racemic mixture is obtained due to the halogenation of
a)
n-pentane
b)
isopentane
c)
neopentane
d)
Both (a) and (b)
|
|
Sreemoyee Choudhury answered |
If free radical halogenation generate a chiral carbon, racemic mixture of halides are always formed due to equal probability of halogenation from both sides of planar free radical.
Consider the following reaction,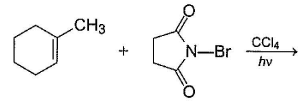 Q. The expected product(s) is/are
Q. The expected product(s) is/are- a)
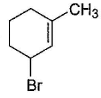
- b)
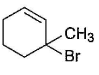
- c)
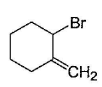
- d)
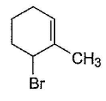
Correct answer is option 'A,B,C,D'. Can you explain this answer?
Consider the following reaction,
Q.
The expected product(s) is/are
a)
b)
c)
d)
|
|
Om Desai answered |
NBS in CCI4 brings about allylic brom ination by free radical mechanism:
The yield of alkyl bromide obtained as a result of heating the dry silver salt of carboxylic acid with bromine in CCI4 is
a) 1°> 3°> 2° bromides
b) 3 ° > 2° > 1°bromides
c) 1°> 2° > 3° bromides
d) 3 ° > 1°> 2 ° bromidesCorrect answer is option 'B'. Can you explain this answer?
|
|
Shraddha Chavan answered |
The reaction follows free radical mechanism and alkyl free radical is formed in the propagation step as
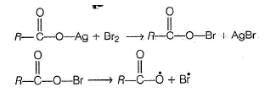
Hence, stability of alkyl free radical (3° > 2° > 1°) determine the reactivity.
Pick out the most reactive alkyl halide for an SN1 reaction.- a)
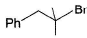
- b)
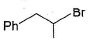
- c)

- d)
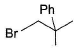
Correct answer is option 'A'. Can you explain this answer?
Pick out the most reactive alkyl halide for an SN1 reaction.
a)
b)
c)
d)
|
|
Vivek Rana answered |
Tertiary halide would be most reactive in SN1 reaction.
Which among the following is true?- a)Freon does not initiate radical chain reactions in stratosphere.
- b)Iodoform initiates radical chain reactions in stratosphere.
- c)Freon initiates radical chain reactions in stratosphere.
- d)Chloroform initiates radical chain reactions in stratosphere.
Correct answer is option 'C'. Can you explain this answer?
Which among the following is true?
a)
Freon does not initiate radical chain reactions in stratosphere.
b)
Iodoform initiates radical chain reactions in stratosphere.
c)
Freon initiates radical chain reactions in stratosphere.
d)
Chloroform initiates radical chain reactions in stratosphere.

|
Learners Habitat answered |
The correct answer is Option C.
Freon (Eg: CFCl3 – Freon11) does initiate radical chain reactions in the stratosphere, which can upset the ozone layer.
Freon (Eg: CFCl3 – Freon11) does initiate radical chain reactions in the stratosphere, which can upset the ozone layer.
Which is the correct formula of Freon-12?- a)CCl2F2
- b)CClF3
- c)CHCl2F
- d)CCl3F
Correct answer is option 'A'. Can you explain this answer?
Which is the correct formula of Freon-12?
a)
CCl2F2
b)
CClF3
c)
CHCl2F
d)
CCl3F

|
Muskan Tanwar answered |
Just a name to be remembered ,nothing to be explained
On reacting ethene with HCl we get:- a)Chloroethane
- b)Chloropropane
- c)Chloromethane
- d)None of these
Correct answer is option 'A'. Can you explain this answer?
On reacting ethene with HCl we get:
a)
Chloroethane
b)
Chloropropane
c)
Chloromethane
d)
None of these

|
Vatturi Anjani answered |
Follows markonikoff rule where the negative group of the compound attaches to the double bond carbon having less no.of hydrogen.
What is the order of reactivity of following halogen acids on alcohols?
HI, HBr, HCl- a)HI < HBr < HCl
- b)HI > HBr > HCl
- c)HI > HCl > HBr
- d)HBr > HCl> HI
Correct answer is option 'B'. Can you explain this answer?
What is the order of reactivity of following halogen acids on alcohols?
HI, HBr, HCl
HI, HBr, HCl
a)
HI < HBr < HCl
b)
HI > HBr > HCl
c)
HI > HCl > HBr
d)
HBr > HCl> HI
|
|
Mira Sharma answered |
That atomic size increases from F to I, so bond length of H-X bond also gets increased and so bond dissociation energy decreases. Hence, ionisation of H-X increases, therefore acidic strength increases in the order
HF < HCl < HBr < HI
So, the reactivity of alcohols towards haloacids is in the order - HI>HBr>HCl
Chlorination of ethane is carried out in presence of- a)anhydrous AlBr3
- b)mercuric chloride
- c)ultraviolet light
- d)zinc chloride
Correct answer is option 'C'. Can you explain this answer?
Chlorination of ethane is carried out in presence of
a)
anhydrous AlBr3
b)
mercuric chloride
c)
ultraviolet light
d)
zinc chloride
|
|
Suresh Iyer answered |
The correct answer is option C
Chlorination of ethane is carried out in the presence of Ultraviolet light to form 1,2-hexachloroethane.
CH3CH3 ----- UVlight ----> CCl3CCl3
So, the correct answer is C.
Chlorination of ethane is carried out in the presence of Ultraviolet light to form 1,2-hexachloroethane.
CH3CH3 ----- UVlight ----> CCl3CCl3
So, the correct answer is C.
The IUPAC name of chloroform is:- a)Dichloromethane
- b)Trichloromethane
- c)Chloromethane
- d)Carbon tetrachloride
Correct answer is option 'B'. Can you explain this answer?
The IUPAC name of chloroform is:
a)
Dichloromethane
b)
Trichloromethane
c)
Chloromethane
d)
Carbon tetrachloride
|
|
Ameya Pillai answered |
**Explanation:**
The IUPAC name of chloroform is trichloromethane. Let's break down the name and understand why it is called trichloromethane.
**1. Basic Structure:**
The basic structure of chloroform consists of a single carbon atom bonded to three chlorine atoms and one hydrogen atom. This can be represented as CHCl3.
**2. Naming Rules:**
According to the IUPAC naming rules for organic compounds, the longest carbon chain in the structure should be identified. In the case of chloroform, there is only one carbon atom, so the longest chain is just one carbon long.
**3. Naming Prefixes:**
The prefixes used in IUPAC nomenclature indicate the number of substituents attached to the main carbon chain. Here, the prefix "tri-" is used because there are three chlorine atoms attached to the carbon atom.
**4. Naming Suffix:**
The suffix in the IUPAC name indicates the functional group present in the compound. Since chloroform contains a single carbon atom bonded to three chlorine atoms, it is classified as a halomethane. Therefore, the suffix used is "-ane."
**Putting it all together:**
Based on the above rules, the IUPAC name for chloroform is trichloromethane. The prefix "tri-" indicates the presence of three chlorine substituents, and the suffix "-ane" indicates that it is a halomethane compound.
Therefore, the correct answer is option **B) Trichloromethane**.
The IUPAC name of chloroform is trichloromethane. Let's break down the name and understand why it is called trichloromethane.
**1. Basic Structure:**
The basic structure of chloroform consists of a single carbon atom bonded to three chlorine atoms and one hydrogen atom. This can be represented as CHCl3.
**2. Naming Rules:**
According to the IUPAC naming rules for organic compounds, the longest carbon chain in the structure should be identified. In the case of chloroform, there is only one carbon atom, so the longest chain is just one carbon long.
**3. Naming Prefixes:**
The prefixes used in IUPAC nomenclature indicate the number of substituents attached to the main carbon chain. Here, the prefix "tri-" is used because there are three chlorine atoms attached to the carbon atom.
**4. Naming Suffix:**
The suffix in the IUPAC name indicates the functional group present in the compound. Since chloroform contains a single carbon atom bonded to three chlorine atoms, it is classified as a halomethane. Therefore, the suffix used is "-ane."
**Putting it all together:**
Based on the above rules, the IUPAC name for chloroform is trichloromethane. The prefix "tri-" indicates the presence of three chlorine substituents, and the suffix "-ane" indicates that it is a halomethane compound.
Therefore, the correct answer is option **B) Trichloromethane**.
Which of the following is not an aryl halide?- a)m-ClCH2C6H4CH2C(CH3)3
- b)(CH3)3CCH2CH3
- c)o-Br-C6H4CH(CH3)CH2CH3
- d)p-ClC6H4CH2CH(CH3)2
Correct answer is 'B'. Can you explain this answer?
Which of the following is not an aryl halide?
a)
m-ClCH2C6H4CH2C(CH3)3
b)
(CH3)3CCH2CH3
c)
o-Br-C6H4CH(CH3)CH2CH3
d)
p-ClC6H4CH2CH(CH3)2
|
|
Ankita Datta answered |
Because it is Open chain compound /Aliphatic Compound and not aryl halide as does not contain halogen atom. it's alkane simply.
Which of the following is the major product of following reaction?
CH3CH = CH2 + HBr in the presence of peroxide gives:- a)2 – bromopropane
- b)1 – bromopropene
- c)1 – bromopropane
- d)2 – bromopropene
Correct answer is option 'C'. Can you explain this answer?
Which of the following is the major product of following reaction?
CH3CH = CH2 + HBr in the presence of peroxide gives:
CH3CH = CH2 + HBr in the presence of peroxide gives:
a)
2 – bromopropane
b)
1 – bromopropene
c)
1 – bromopropane
d)
2 – bromopropene
|
|
Garima Kunwar answered |
In presence of HBr and peroxide anti markonikov's addition in supported. Hence 1-bromopropane will we major product
Exposure to lower levels of a compound in air can lead to slightly impaired hearing and vision. Identify the compound.- a)Chloroform
- b)Chloromethane
- c)Carbon tetrachloride
- d)Methylene chloride
Correct answer is option 'D'. Can you explain this answer?
Exposure to lower levels of a compound in air can lead to slightly impaired hearing and vision. Identify the compound.
a)
Chloroform
b)
Chloromethane
c)
Carbon tetrachloride
d)
Methylene chloride
|
|
Lavanya Menon answered |
The correct answer is Option D.
Methylene chloride (Dichloromethane) Dichloromethane, also known as methylene chloride, is one of the polyhalogen compounds present in the form of solvent. Low levels of methylene chloride exposure in the air can cause slight hearing and vision impairment.
Comprehension TypeDirection (Q. Nos. 13-15) This section contains a paragraph, describing theory, experiments, data, etc.
Three questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).PassageAn alcohol (R — OH) can be converted into alkyl chloride by the treatm ent with HCI. Reaction involves protonation of alcohol followed by the formation of carbocation intermediate. Carbocation intermediate in the final step undergo nucleophilic attack by Cl- ion as :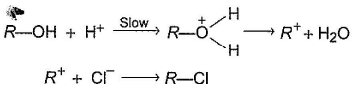 Q. Which of the following alcohol reacts most easily?
Q. Which of the following alcohol reacts most easily?- a)
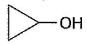
- b)
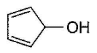
- c)
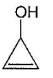
- d)
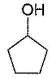
Correct answer is 'C'. Can you explain this answer?
Comprehension Type
Direction (Q. Nos. 13-15) This section contains a paragraph, describing theory, experiments, data, etc.
Three questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).
Three questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).
Passage
An alcohol (R — OH) can be converted into alkyl chloride by the treatm ent with HCI. Reaction involves protonation of alcohol followed by the formation of carbocation intermediate. Carbocation intermediate in the final step undergo nucleophilic attack by Cl- ion as :
Q.
Which of the following alcohol reacts most easily?
a)
b)
c)
d)
|
|
Om Desai answered |
As mentioned in mechanism, reaction proceed via carbocation intermediate. Hence, alcohol forming most stable carbocation reacts most easily. Alcohol (c) forms aromatic (highly stable) carbocation, hence most reactive.
The most common freons in industrial use is manufactured by
- a)Wurtz reaction
- b)Sandmeyer reaction
- c)Fittig reaction
- d)By Swarts reaction
Correct answer is option 'D'. Can you explain this answer?
The most common freons in industrial use is manufactured by
a)
Wurtz reaction
b)
Sandmeyer reaction
c)
Fittig reaction
d)
By Swarts reaction
|
|
Rajesh Gupta answered |
Most common Freon is synthesized by Swartz reaction.
The correct answer is:
The correct answer is:
4. By Swarts reaction
Explanation:
-
Swarts reaction is commonly used for the manufacture of freons (chlorofluorocarbons, CFCs), where halogen atoms in a molecule are replaced by fluorine atoms using antimony trifluoride (SbF3) or similar reagents.
-
Wurtz reaction: Typically used to couple two alkyl halides to form a longer carbon chain.
-
Sandmeyer reaction: Used for the substitution of an aryl diazonium salt with a halogen or other group.
-
Fittig reaction: Used to couple aryl halides to form biaryls.
So, Swarts reaction is the correct choice for the production of freons.
Central nervous system can be depressed by the use of which of the following:- a)
- Chloroform
- b)Freon
- c)Iodoform
- d)DDT
Correct answer is option 'A'. Can you explain this answer?
Central nervous system can be depressed by the use of which of the following:
a)
- Chloroform
b)
Freon
c)
Iodoform
d)
DDT
|
|
Priya Patel answered |
Chloroform, or trichloromethane, is an organic compound with formula CHCl3. It is a colorless, sweet-smelling, dense liquid that is produced on a large scale as a precursor to PTFE. It is also a precursor to various refrigerants. It is one of the four chloromethanes and a trihalomethane. It is a powerful anesthetic, euphoriant, anxiolytic and sedative when inhaled or ingested.
Which is incorrect about Hunsdiecker's reaction ?- a)Only Cl2 can give alkyl halide
- b)I2 will give ester when treated with RCOOAg
- c)The reaction proceeds through free radical
- d)F2 cannot give alkyl halide
Correct answer is option 'A'. Can you explain this answer?
Which is incorrect about Hunsdiecker's reaction ?
a)
Only Cl2 can give alkyl halide
b)
I2 will give ester when treated with RCOOAg
c)
The reaction proceeds through free radical
d)
F2 cannot give alkyl halide
|
|
Priya Chavan answered |
Except F2, almost all halogens react with RCOOAg giving alkyl halide via Hunsdiecker reaction. With l2 if RCOOAg is in excess, R— I formed in first step reacts further with unreacted salt to give ester as
R — COOAg+ R — I → R — COOR + AgI
R — COOAg+ R — I → R — COOR + AgI
Only One Option Correct TypeDirection (Q. Nos. 1-8) This section contains 8 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct.Q. Which of the SN2 reaction is fastest?- a)
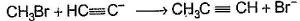
- b)
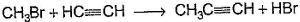
- c)
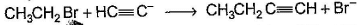
- d)
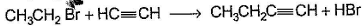
Correct answer is option 'A'. Can you explain this answer?
Only One Option Correct Type
Direction (Q. Nos. 1-8) This section contains 8 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct.
Q.
Which of the SN2 reaction is fastest?
a)
b)
c)
d)
|
|
Monika Devi answered |
SN2 reaction means this will be applicable for primary or 1 degree carbon atom and attack by the reagent is forwarded so on doing mechanism we getted that CH3 carry + charge & Br get - charge so acetyl group that have negative charge goes on methyl group & Br react with electronic species
Which of the following gives the same substitution (SN2) product with C2H5Br no matter whether sodium or silver salt is used? - a)CN-
- b)OCN-
- c)SCN-
- d)
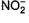
Correct answer is option 'B,D'. Can you explain this answer?
Which of the following gives the same substitution (SN2) product with C2H5Br no matter whether sodium or silver salt is used?
a)
CN-
b)
OCN-
c)
SCN-
d)
|
|
Nikita Singh answered |
Both donate lone pair from better donor nitrogen atom.
One Integer Value Correct TypeDirection (Q. Nos. 18-20) This section contains 3 questions. When worked out will result in an integer from 0 to 9 (both inclusive).Consider the following reaction,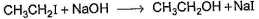 Q. The above reaction was started taking equal concentrations of ethyl iodide and NaOH. After 1.0 h, concentration of iodoethane is dropped to (1/3)rd of initial value. By what factor, the rate of reaction would have been decreased by the same time?
Q. The above reaction was started taking equal concentrations of ethyl iodide and NaOH. After 1.0 h, concentration of iodoethane is dropped to (1/3)rd of initial value. By what factor, the rate of reaction would have been decreased by the same time?
Correct answer is '9'. Can you explain this answer?
One Integer Value Correct Type
Direction (Q. Nos. 18-20) This section contains 3 questions. When worked out will result in an integer from 0 to 9 (both inclusive).
Consider the following reaction,
Q.
The above reaction was started taking equal concentrations of ethyl iodide and NaOH. After 1.0 h, concentration of iodoethane is dropped to (1/3)rd of initial value. By what factor, the rate of reaction would have been decreased by the same time?
|
|
Priyanka Sharma answered |
Rate = A [C2H5I] [OH-] Rate is linear function of both alkyl halide and nucleophile concentration both will decrease by same factor. Hence, if after 1.0 h concentration of iodoethane decreases to 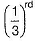 concentration of hydroxide will also decrease by the same factor and rate by
concentration of hydroxide will also decrease by the same factor and rate by 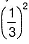 , i.e. by 9 times.
, i.e. by 9 times.
Pick out the compound which reacts fastest in the presence of AgNO3.- a)(CH3)3CCI
- b)(CH3)2CHCH2CI
- c)(CH3)2CHCI
- d)CH3CH2CI
Correct answer is option 'A'. Can you explain this answer?
Pick out the compound which reacts fastest in the presence of AgNO3.
a)
(CH3)3CCI
b)
(CH3)2CHCH2CI
c)
(CH3)2CHCI
d)
CH3CH2CI

|
Tarun Chakraborty answered |
Tertiary halide would react at fastest rate with AgNO3 as reaction will proceed by SN1 mechanism.
Depletion of Ozone is believed to increase:- a)Population
- b)Ultraviolet rays
- c)Pollution
- d)Greenery
Correct answer is option 'B'. Can you explain this answer?
Depletion of Ozone is believed to increase:
a)
Population
b)
Ultraviolet rays
c)
Pollution
d)
Greenery
|
|
Anshita Singh answered |
As we know, ozone layer is present in the upper layer of atmosphere i.e. troposphere which acts as a shield to protect us from uv rays...but due to more and more production of cfcs ozone is depleting due to which it is believed that the amount of uv rays is increasing
Which of the following is formed by Swarts Reaction?- a)Alkyl iodide
- b)Alkyl fluoride
- c)Alkyl bromide
- d)Alkyl chloride
Correct answer is option 'B'. Can you explain this answer?
Which of the following is formed by Swarts Reaction?
a)
Alkyl iodide
b)
Alkyl fluoride
c)
Alkyl bromide
d)
Alkyl chloride
|
|
Rohit Shah answered |
In Swarts’ reaction, heavy metal fluorides are used as reagents. It is a reaction in which alkyl fluorides are formed when alkyl bromide or chloride reacts with metal fluorides. In the reaction, higher alkyl halide(R-Cl) react with lower metal halide (AgF) to form lower alkyl halide(R-F). If we don’t use heavy metal fluoride F ion cannot displace Cl/Br. The reaction will not occur or it may form some other compound.
Bromomethane, Chloromethane, Dibromomethane, 1 – Chloropropane, Isopropyl chloride, 1 – Chlorobutaneare all- a)Slightly soluble in organic solvents
- b)Completely soluble in water
- c)Completely soluble in organic solvents
- d)Insoluble in organic solvents
Correct answer is option 'C'. Can you explain this answer?
Bromomethane, Chloromethane, Dibromomethane, 1 – Chloropropane, Isopropyl chloride, 1 – Chlorobutaneare all
a)
Slightly soluble in organic solvents
b)
Completely soluble in water
c)
Completely soluble in organic solvents
d)
Insoluble in organic solvents

|
Saptarshi Ghoshal answered |
These all are covalent compounds hence are soluble in organic solvents.
Pick up the correct statement about alkyl halides.- a)They are associated with each other by H-bonds.
- b)They dissolve easily in organic solvents.
- c)They dissolve in water quickly.
- d)They do not contain any polar bond in their molecules.
Correct answer is option 'B'. Can you explain this answer?
Pick up the correct statement about alkyl halides.
a)
They are associated with each other by H-bonds.
b)
They dissolve easily in organic solvents.
c)
They dissolve in water quickly.
d)
They do not contain any polar bond in their molecules.
|
|
Preeti Iyer answered |
In general organic compounds are soluble in organic solvents. Hence alkyl halides are soluble in organic solvents.
Addition of bromine on propene in the presence of brine yields a mixture of- a)CH3CHCICH2Br and CH3CHBrCH2CI
- b)CH3CHCICH2Br and CH3CHBrCH2Br
- c)CH3CHCICH2CI and CH3CHBrCH2Br
- d)CH3CHCICH2Cl and CH3CHBrCH2Cl
Correct answer is option 'C'. Can you explain this answer?
Addition of bromine on propene in the presence of brine yields a mixture of
a)
CH3CHCICH2Br and CH3CHBrCH2CI
b)
CH3CHCICH2Br and CH3CHBrCH2Br
c)
CH3CHCICH2CI and CH3CHBrCH2Br
d)
CH3CHCICH2Cl and CH3CHBrCH2Cl
|
|
Snehal Iyer answered |
Nucleophilic attack in step-ll occur at the carbon atom which can better accommodate the positive charge. Hence, attack of Br- or Cl- in second step occur at 2° carbon rather that at 1° carbon.
In dehydrohalogenation reactions the preferred product is that alkene which has the greatest number of alkyl groups attached to the doubly bonded carbon atoms. This rule is known as:- a)Saytzeff’s rule
- b)Markoniknov’s rule
- c)Anti markoniknov’s rule
- d)None of these
Correct answer is option 'A'. Can you explain this answer?
In dehydrohalogenation reactions the preferred product is that alkene which has the greatest number of alkyl groups attached to the doubly bonded carbon atoms. This rule is known as:
a)
Saytzeff’s rule
b)
Markoniknov’s rule
c)
Anti markoniknov’s rule
d)
None of these
|
|
Vijay Bansal answered |
In dehydrohalogenation reactions the preferred product is that alkene which has the greatest number of alkyl groups attached to the doubly bonded carbon atoms. This rule is also known as Saytzeff’s rule.
Chapter doubts & questions for Haloalkanes and Haloarenes - Science for ACT 2025 is part of ACT exam preparation. The chapters have been prepared according to the ACT exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for ACT 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Haloalkanes and Haloarenes - Science for ACT in English & Hindi are available as part of ACT exam.
Download more important topics, notes, lectures and mock test series for ACT Exam by signing up for free.
Science for ACT
486 videos|517 docs|337 tests
|
Signup to see your scores go up within 7 days!
Study with 1000+ FREE Docs, Videos & Tests
10M+ students study on EduRev

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Forgot Password
OR
Signup to see your scores
go up within 7 days!
Access 1000+ FREE Docs, Videos and Tests
Takes less than 10 seconds to signup