All Exams >
JAMB >
Chemistry for JAMB >
All Questions
All questions of Acids, Bases & Salts for JAMB Exam
Acid turns blue litmusA: OrangeB: PinkC: greenD: RedCorrect answer is 'D'. Can you explain this answer?
Acid turns blue litmus
A: Orange
B: Pink
C: green
D: Red

|
Jithin Chawla answered |
Acids turn blue litmus red. Litmus paper is used as indicator to check acids and bases.
Which of the following is a natural indicator- a)Turmeric
- b)Methyl orange
- c)Oxalic acid.
- d)Phenolphthalein
Correct answer is option 'A'. Can you explain this answer?
Which of the following is a natural indicator
a)
Turmeric
b)
Methyl orange
c)
Oxalic acid.
d)
Phenolphthalein

|
Sanjana Kumar answered |
Some of the natural indicators are red cabbage juice, onion paste, turmeric, lemon juice etc. Natural indicators are turmric,litmus,china rose etc are some natural indicators. Some Natural Indicators are red Cabbage juice,turmeric,china rose[hibiscus] and litmus.
For HF, pKa = 3.45. What is the pH of an aqueous buffer solution that is 0.1M HF (aq) and 0.300 M KF (aq)?
a)11.03b)2.97c)10.07d)3.93 Correct answer is option 'D'. Can you explain this answer?
|
|
Suresh Iyer answered |
Given, pKa = 3.45
Concentration of HF = 0.1 M, concentration of KF = 0.300 M
For acidic buffer;
pH = pKa + log [salt of weak acid]/[weak acid]
= 3.45 + log0.3/0.1
= 3.45 + 0.48
= 3.93
Concentration of HF = 0.1 M, concentration of KF = 0.300 M
For acidic buffer;
pH = pKa + log [salt of weak acid]/[weak acid]
= 3.45 + log0.3/0.1
= 3.45 + 0.48
= 3.93
Consider KA, the potassium salt of a weak acid, HA. If a sample of KA is dissolved in water, with no other substances added, which of the following statements is true (approximately)?- a)[H3O+] = [OH-]
- b)[HA] = [K+]
- c)[HA] = [OH-]
- d)[H3O+] = [A-]
Correct answer is option 'C'. Can you explain this answer?
Consider KA, the potassium salt of a weak acid, HA. If a sample of KA is dissolved in water, with no other substances added, which of the following statements is true (approximately)?
a)
[H3O+] = [OH-]
b)
[HA] = [K+]
c)
[HA] = [OH-]
d)
[H3O+] = [A-]
|
|
Suresh Iyer answered |
The correct answer is option C
[HA] = [OH–]
The reaction is: A– + H2O ⇌ HA + OH–
[HA] = [OH–]
The reaction is: A– + H2O ⇌ HA + OH–
What is the final color of lemon juice when tested with China rose indicator?- a)Dark pink (magenta)
- b)Green
- c)No change
- d)Blue
Correct answer is option 'A'. Can you explain this answer?
a)
Dark pink (magenta)
b)
Green
c)
No change
d)
Blue

|
Rainbow Rise Classes answered |
Lemon juice is acidic, so when tested with China rose indicator, it will turn dark pink (magenta). This color change indicates the acidic nature of the solution, as China rose turns acidic solutions to dark pink.
What new substance is formed as a result of a neutralization reaction between hydrochloric acid and sodium hydroxide?- a)Carbon dioxide
- b)Sodium chloride
- c)Oxygen gas
- d)Hydrogen gas
Correct answer is option 'B'. Can you explain this answer?
What new substance is formed as a result of a neutralization reaction between hydrochloric acid and sodium hydroxide?
a)
Carbon dioxide
b)
Sodium chloride
c)
Oxygen gas
d)
Hydrogen gas

|
Rainbow Rise Classes answered |
In the neutralization reaction between hydrochloric acid (HCl) and sodium hydroxide (NaOH), the new substance formed is sodium chloride (NaCl), commonly known as table salt. This salt is produced along with water (H2O) as the products of the reaction, highlighting the typical outcome of a neutralization process between an acid and a base.
Role of NH4Cl in qualitative analysis of third group cations- a)to provide basic medium
- b)to provide acidic medium
- c)to increase the degree of dissociation of NH4OH
- d)to suppress the degree of dissociation of NH4OH
Correct answer is option 'D'. Can you explain this answer?
Role of NH4Cl in qualitative analysis of third group cations
a)
to provide basic medium
b)
to provide acidic medium
c)
to increase the degree of dissociation of NH4OH
d)
to suppress the degree of dissociation of NH4OH
|
|
Raghav Bansal answered |
The correct answer is option D
Common ion effect is observed when a solution of weak electrolyte is mixed with a solution of strong electrolyte, which provides an ion common to that provided by weak electrolyte.
The NH4OH is a weak base and it does not ionise completely. Thus, due to presence of common ion NH4+ in NH4Cl, it suppresses the ionisation of weak base NH4OH in order to decrease the OH- concentration so that higher group cations will not get precipitated.
Thus the pair NH4OH+NH4Cl shows a common ion effect.
Ammonium chloride suppresses the ionization of ammonium hydroxide
.
Common ion effect is observed when a solution of weak electrolyte is mixed with a solution of strong electrolyte, which provides an ion common to that provided by weak electrolyte.
The NH4OH is a weak base and it does not ionise completely. Thus, due to presence of common ion NH4+ in NH4Cl, it suppresses the ionisation of weak base NH4OH in order to decrease the OH- concentration so that higher group cations will not get precipitated.
Thus the pair NH4OH+NH4Cl shows a common ion effect.
Ammonium chloride suppresses the ionization of ammonium hydroxide
.
We eat a variety of foods still pH of our blood does not change every time. The reason is- a)Strong bases in the blood donot let pH change
- b)Stomach wall is resistant
- c)There are buffers in the blood which resist pH change
- d)Strong acids in the blood donot let pH change
Correct answer is option 'C'. Can you explain this answer?
We eat a variety of foods still pH of our blood does not change every time. The reason is
a)
Strong bases in the blood donot let pH change
b)
Stomach wall is resistant
c)
There are buffers in the blood which resist pH change
d)
Strong acids in the blood donot let pH change
|
|
Pooja Shah answered |
The correct answer is option C
pH of blood remains constant because of the buffer system present in the blood. Acid-base buffers confer resistance to a change in the pH of a solution when hydrogen ions (protons) or hydroxide ions are added or removed. An acid-base buffer typically consists of a weak acid, and its conjugate base (salt).It used to neutralized the extra added protons or OH- in blood.The buffer for maintaining acid-base balance in the blood is the carbonic-acid-bicarbonate buffer.So pH of blood remains even after eating spicy food.
pH of blood remains constant because of the buffer system present in the blood. Acid-base buffers confer resistance to a change in the pH of a solution when hydrogen ions (protons) or hydroxide ions are added or removed. An acid-base buffer typically consists of a weak acid, and its conjugate base (salt).It used to neutralized the extra added protons or OH- in blood.The buffer for maintaining acid-base balance in the blood is the carbonic-acid-bicarbonate buffer.So pH of blood remains even after eating spicy food.
Which of the following acid is present in Tomato?- a)Oxalic acid
- b)Tartaric acid
- c)Phosphoric acid
- d)Lactic acid
Correct answer is option 'A'. Can you explain this answer?
Which of the following acid is present in Tomato?
a)
Oxalic acid
b)
Tartaric acid
c)
Phosphoric acid
d)
Lactic acid

|
Jambada Krishnamurthy answered |
Actually tomato contains oxalic acid.
tamarind contains tartaric acid
phosphoric is used for fertilizers, detergents and soft drinks
lactic acid is produced when you do exercises i.e the muscle cells and red blood cells produces lactic acid
so we can conclude that oxalic acid is present in tomato
tamarind contains tartaric acid
phosphoric is used for fertilizers, detergents and soft drinks
lactic acid is produced when you do exercises i.e the muscle cells and red blood cells produces lactic acid
so we can conclude that oxalic acid is present in tomato
If the value of the solubility product for AgBr is 4.0 x 10-12 at 25°C, calculate the solubility of AgBr(s) in water.- a)2.5 x 10-13
- b)2.5 x 10-6
- c)2 x 10-6
- d)5.0 x10-7
Correct answer is option 'C'. Can you explain this answer?
If the value of the solubility product for AgBr is 4.0 x 10-12 at 25°C, calculate the solubility of AgBr(s) in water.
a)
2.5 x 10-13
b)
2.5 x 10-6
c)
2 x 10-6
d)
5.0 x10-7
|
|
Raghav Bansal answered |
Ksp = 4.0 x 10-12
For AB type salt, Solubility = Ksp1/2
= (4.0 x 10-12)1/2
= 2 x 10-6
For AB type salt, Solubility = Ksp1/2
= (4.0 x 10-12)1/2
= 2 x 10-6
The following equilibrium exists in aqueous solutionCH3COOH 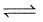 CH3COO- + H+
CH3COO- + H+
If dilute HCl is added:- a)Acetate ion concentration will increase
- b)Acetate ion concentration will decrease
- c)The equilibrium constant will decrease
- d)The equilibrium constant will increase.
Correct answer is option 'B'. Can you explain this answer?
The following equilibrium exists in aqueous solution
CH3COOH 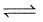 CH3COO- + H+
CH3COO- + H+
If dilute HCl is added:
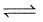 CH3COO- + H+
CH3COO- + H+If dilute HCl is added:
a)
Acetate ion concentration will increase
b)
Acetate ion concentration will decrease
c)
The equilibrium constant will decrease
d)
The equilibrium constant will increase.
|
|
Sahana Marigoudar answered |
H+ concentration increases which makes the reaction to proceed through reverse direction due to which acetic acid concentration increases.
State whether the following statement is True or FalseAcids taste sour and bases taste bitter.- a)True
- b)False
Correct answer is option 'A'. Can you explain this answer?
State whether the following statement is True or False
Acids taste sour and bases taste bitter.
a)
True
b)
False

|
Bespoke Classes answered |
- True! Acids like curd, lemon juice, orange juice, and vinegar taste sour because they contain acids.
- On the other hand, bases like baking soda taste bitter.
- So, acids usually have a sour taste, while bases have a bitter taste.
- When you taste something sour like lemon juice, it might have acids in it.
- And if something tastes bitter like baking soda, it could be a base.
- Remember, sour for acids and bitter for bases!
What does phenolphthalein indicate when the solution is basic?- a)It turns dark pink
- b)It turns green
- c)It remains colourless
- d)It turns blue
Correct answer is option 'A'. Can you explain this answer?
What does phenolphthalein indicate when the solution is basic?
a)
It turns dark pink
b)
It turns green
c)
It remains colourless
d)
It turns blue

|
Vp Classes answered |
When phenolphthalein is added to a basic solution, it changes its color to dark pink. This change helps us know if a solution is basic or not. So, when you see the pink color, you can tell that the solution is basic.
What is the pH nature of distilled water?- a)Acidic
- b)Basic
- c)Neutral
- d)Saline
Correct answer is option 'C'. Can you explain this answer?
a)
Acidic
b)
Basic
c)
Neutral
d)
Saline

|
Coachify answered |
Distilled water is neutral, with a pH of around 7. It does not change the color of either blue or red litmus paper, which signifies its neutral nature.
What happens in a neutralization reaction?- a)An acid reacts with another acid to form a base and water.
- b)A base reacts with another base to form an acid and water.
- c)An acid reacts with a base to form salt and water.
- d)An acid reacts with a base to form only salt.
Correct answer is option 'C'. Can you explain this answer?
a)
An acid reacts with another acid to form a base and water.
b)
A base reacts with another base to form an acid and water.
c)
An acid reacts with a base to form salt and water.
d)
An acid reacts with a base to form only salt.

|
Vp Classes answered |
In a neutralization reaction, an acid reacts with a base to form salt and water. This process is characterized by the combination of H⁺ ions from the acid and OH⁻ ions from the base to form water (H₂O), and the remaining ions form a salt.
When the value of Kc is very small then- a)reaction is at start
- b)reactant conc. Is maximum
- c)reactant conc. Is minimum
- d)reaction is completed
Correct answer is option 'B'. Can you explain this answer?
When the value of Kc is very small then
a)
reaction is at start
b)
reactant conc. Is maximum
c)
reactant conc. Is minimum
d)
reaction is completed
|
|
Rutuja Desai answered |
When the value of Kc is very small, it indicates that the equilibrium position of the reaction lies towards the left side, meaning that the concentration of the reactants is relatively high compared to the concentration of the products.
Let's understand this in detail:
Equilibrium Constant (Kc):
The equilibrium constant, Kc, is a measure of the extent of a chemical reaction at equilibrium. It is defined as the ratio of the product concentrations to the reactant concentrations, each raised to their respective stoichiometric coefficients, at equilibrium, with each concentration term raised to the power equal to its stoichiometric coefficient in the balanced chemical equation.
Kc = [Products]^n / [Reactants]^m
Where [Products] and [Reactants] represent the concentrations of the products and reactants at equilibrium, respectively, and n and m are the stoichiometric coefficients of the products and reactants, respectively.
Interpretation of a Very Small Kc Value:
When the value of Kc is very small, it means that the numerator (concentration of products) is much smaller compared to the denominator (concentration of reactants).
This implies that the concentration of the reactants is significantly higher than the concentration of the products at equilibrium.
Explanation of Correct Answer (Option B):
The correct answer, "reactant conc. is maximum," indicates that when the value of Kc is very small, the concentration of the reactants is at its maximum.
This is because the equilibrium position favors the reactants, and the reaction is not proceeding significantly towards the product side. As a result, the concentration of the reactants remains high, while the concentration of the products is relatively low.
In other words, the reaction is not proceeding to a significant extent, and the reactants are dominant in the equilibrium mixture.
Therefore, when the value of Kc is very small, it signifies that the reaction is at the start, and the concentration of the reactants is maximum.
Let's understand this in detail:
Equilibrium Constant (Kc):
The equilibrium constant, Kc, is a measure of the extent of a chemical reaction at equilibrium. It is defined as the ratio of the product concentrations to the reactant concentrations, each raised to their respective stoichiometric coefficients, at equilibrium, with each concentration term raised to the power equal to its stoichiometric coefficient in the balanced chemical equation.
Kc = [Products]^n / [Reactants]^m
Where [Products] and [Reactants] represent the concentrations of the products and reactants at equilibrium, respectively, and n and m are the stoichiometric coefficients of the products and reactants, respectively.
Interpretation of a Very Small Kc Value:
When the value of Kc is very small, it means that the numerator (concentration of products) is much smaller compared to the denominator (concentration of reactants).
This implies that the concentration of the reactants is significantly higher than the concentration of the products at equilibrium.
Explanation of Correct Answer (Option B):
The correct answer, "reactant conc. is maximum," indicates that when the value of Kc is very small, the concentration of the reactants is at its maximum.
This is because the equilibrium position favors the reactants, and the reaction is not proceeding significantly towards the product side. As a result, the concentration of the reactants remains high, while the concentration of the products is relatively low.
In other words, the reaction is not proceeding to a significant extent, and the reactants are dominant in the equilibrium mixture.
Therefore, when the value of Kc is very small, it signifies that the reaction is at the start, and the concentration of the reactants is maximum.
A mixture of NH4Cl and NH4OH shows no change in pH upon addition small amount of HCl. This is because it is- a)A neutral solution
- b)A buffer
- c)A basic solution due to presence of NH4OH
- d)An acidic solution due to presence of NH4Cl
Correct answer is option 'B'. Can you explain this answer?
A mixture of NH4Cl and NH4OH shows no change in pH upon addition small amount of HCl. This is because it is
a)
A neutral solution
b)
A buffer
c)
A basic solution due to presence of NH4OH
d)
An acidic solution due to presence of NH4Cl
|
|
Rajat Patel answered |
A mixture of weak base and its salt with a strong acid serves as an basic buffer which resists changes in pH upon addition of small amount of acid or base.
Which of the following is a characteristic property of bases?- a)Sour taste
- b)Ability to turn blue litmus paper red
- c)Ability to conduct electricity
- d)Reactivity with metals to produce hydrogen gas
Correct answer is option 'C'. Can you explain this answer?
Which of the following is a characteristic property of bases?
a)
Sour taste
b)
Ability to turn blue litmus paper red
c)
Ability to conduct electricity
d)
Reactivity with metals to produce hydrogen gas

|
Sun Ray Institute answered |
Bases are ionic compounds that dissociate in water to produce OH- ions, which enable them to conduct electricity.
Which of the following is an example of basic buffer?- a)A mixture of HCl and NaOH
- b)A mixture of NH4Cl and NH4OH
- c)A mixture of CH3COOH and OH3COONa
- d)1M solution of NaOH
Correct answer is option 'B'. Can you explain this answer?
Which of the following is an example of basic buffer?
a)
A mixture of HCl and NaOH
b)
A mixture of NH4Cl and NH4OH
c)
A mixture of CH3COOH and OH3COONa
d)
1M solution of NaOH
|
|
Nandini Iyer answered |
Acidic buffer solution contains equimolar quantities of a weak acid and its salt with strong base. For example: an acetic acid, CH3COOH and sodium acetate I.e. CH3COONa. Basic buffer solution contains equimolar quantities of a weak base and its salt with strong acid.
Which acid is commonly found in vinegar?- a)Acetic acid
- b)Citric acid
- c)Lactic acid
- d)Oxalic acid
Correct answer is option 'A'. Can you explain this answer?
Which acid is commonly found in vinegar?
a)
Acetic acid
b)
Citric acid
c)
Lactic acid
d)
Oxalic acid

|
Gunjan Lakhani answered |
Acetic acid is the acid commonly found in vinegar. It is a weak acid that gives vinegar its characteristic sour taste and pungent smell. Acetic acid is also widely used in the food industry for pickling and preserving food items.
What color does litmus paper turn when added to an acidic solution?- a)Green
- b)Red
- c)Blue
- d)Yellow
Correct answer is option 'B'. Can you explain this answer?
What color does litmus paper turn when added to an acidic solution?
a)
Green
b)
Red
c)
Blue
d)
Yellow
|
|
Subset Academy answered |
Litmus paper turns red when added to an acidic solution. This color change is a result of the acidic nature of the solution, which causes the litmus paper to shift its color to red. It is a simple and effective way to test for acidity using a natural indicator like litmus.
What is the product when an acid reacts with a metal?- a)Salt and water
- b)Salt and hydrogen gas
- c)Salt and carbon dioxide gas
- d)Salt and oxygen gas
Correct answer is option 'B'. Can you explain this answer?
What is the product when an acid reacts with a metal?
a)
Salt and water
b)
Salt and hydrogen gas
c)
Salt and carbon dioxide gas
d)
Salt and oxygen gas

|
Sun Ray Institute answered |
When an acid reacts with a metal, the products are a salt (ionic compound) and hydrogen gas.
Which of the following substances is an acid?- a)Sodium chloride
- b)Sodium hydroxide
- c)Hydrochloric acid
- d)Calcium carbonate
Correct answer is option 'C'. Can you explain this answer?
Which of the following substances is an acid?
a)
Sodium chloride
b)
Sodium hydroxide
c)
Hydrochloric acid
d)
Calcium carbonate

|
Sun Ray Institute answered |
Hydrochloric acid is a strong acid that dissociates in water to produce H3O+ ions.
Which of the following salts undergoes hydrolysis?- a)Sodium chloride
- b)Sodium sulfate
- c)Sodium carbonate
- d)Sodium nitrate
Correct answer is option 'C'. Can you explain this answer?
Which of the following salts undergoes hydrolysis?
a)
Sodium chloride
b)
Sodium sulfate
c)
Sodium carbonate
d)
Sodium nitrate

|
Sun Ray Institute answered |
Sodium carbonate (Na2CO3) undergoes hydrolysis in water, resulting in the formation of sodium hydroxide and carbonic acid.
Which acid is commonly found in vinegar?- a)Hydrochloric acid
- b)Nitric acid
- c)Citric acid
- d)Sulfuric acid
Correct answer is option 'C'. Can you explain this answer?
Which acid is commonly found in vinegar?
a)
Hydrochloric acid
b)
Nitric acid
c)
Citric acid
d)
Sulfuric acid

|
Sun Ray Institute answered |
Citric acid is a naturally occurring organic acid found in fruits, particularly citrus fruits such as lemons and oranges.
What is the pH value of a neutral solution?- a)0
- b)7
- c)14
- d)It can vary
Correct answer is option 'B'. Can you explain this answer?
What is the pH value of a neutral solution?
a)
0
b)
7
c)
14
d)
It can vary

|
Sun Ray Institute answered |
The pH scale ranges from 0 to 14, with 7 representing a neutral solution. A pH of 7 indicates equal concentrations of H+ and OH- ions.
What is the function of acid/base indicators?- a)To determine the pH of a solution
- b)To neutralize acids and bases
- c)To conduct electricity
- d)To test the solubility of salts
Correct answer is option 'A'. Can you explain this answer?
What is the function of acid/base indicators?
a)
To determine the pH of a solution
b)
To neutralize acids and bases
c)
To conduct electricity
d)
To test the solubility of salts

|
Sun Ray Institute answered |
Acid/base indicators are substances that change color depending on the pH of the solution, allowing us to determine whether the solution is acidic, basic, or neutral.
What is the process of salt formation by combining an acid and a base called?- a)Neutralization
- b)Oxidation
- c)Precipitation
- d)Hydrolysis
Correct answer is option 'A'. Can you explain this answer?
What is the process of salt formation by combining an acid and a base called?
a)
Neutralization
b)
Oxidation
c)
Precipitation
d)
Hydrolysis

|
Sun Ray Institute answered |
Neutralization is the chemical reaction between an acid and a base to form a salt and water.
What is the basicity of an acid?- a)The number of hydrogen ions it donates
- b)The ability to conduct electricity
- c)The pH value of its solution
- d)The number of hydroxide ions it produces
Correct answer is option 'A'. Can you explain this answer?
What is the basicity of an acid?
a)
The number of hydrogen ions it donates
b)
The ability to conduct electricity
c)
The pH value of its solution
d)
The number of hydroxide ions it produces

|
Sun Ray Institute answered |
The basicity of an acid refers to the number of hydrogen ions (H+) it donates when dissolved in water.
Which of the following is an example of a double salt?- a)Sodium chloride
- b)Calcium sulfate
- c)Potassium nitrate
- d)Potassium alum
Correct answer is option 'D'. Can you explain this answer?
Which of the following is an example of a double salt?
a)
Sodium chloride
b)
Calcium sulfate
c)
Potassium nitrate
d)
Potassium alum

|
Sun Ray Institute answered |
Alums are examples of double salts that contain a combination of sulfate and other ions.
A mixture of CH3COOH and CH3COONa behaves as- a)Basic buffer
- b)Ionic buffer
- c)Acidic buffer
- d)Neutral buffer
Correct answer is option 'C'. Can you explain this answer?
A mixture of CH3COOH and CH3COONa behaves as
a)
Basic buffer
b)
Ionic buffer
c)
Acidic buffer
d)
Neutral buffer
|
|
Pooja Mehta answered |
CH3COONH4 can act as a buffer solution as:-
When a strong acid is added to the solution, all the H+ ions take up CH3COO- and form acetic acid, CH3COOH which is a weakly dissociating acid.
When a strong base is added to the solution, all the OH- ions take NH4+ and form ammonium hydroxide, NH4OH which is a weakly dissociating base.
And we know that the salt of a weak acid and a weak base acts as buffer
Chapter doubts & questions for Acids, Bases & Salts - Chemistry for JAMB 2025 is part of JAMB exam preparation. The chapters have been prepared according to the JAMB exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for JAMB 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Acids, Bases & Salts - Chemistry for JAMB in English & Hindi are available as part of JAMB exam.
Download more important topics, notes, lectures and mock test series for JAMB Exam by signing up for free.
Chemistry for JAMB
213 videos|209 docs|162 tests
|

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup on EduRev and stay on top of your study goals
10M+ students crushing their study goals daily









