All Exams >
JAMB >
Chemistry for JAMB >
All Questions
All questions of Energy Changes for JAMB Exam
In which of the following cases, entropy of I is larger than that of II?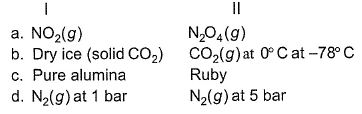
- a)a
- b)b
- c)c
- d)d
Correct answer is option 'D'. Can you explain this answer?
In which of the following cases, entropy of I is larger than that of II?
a)
a
b)
b
c)
c
d)
d

|
Infinity Academy answered |
a) More molar mass, more entropy. So ∆SN2O4 > ∆SNO2
b) CO2 has more entropy than dry ice at -78°C
c) Pure alumina iss crystalline solid while ruby is amorphous. And ∆Samorphous > ∆SCrystalline. So alumina has less entropy than ruby.
d) At lower pressure, entropy be higher as gas particles are far from each other. So (∆SN2)1 bar > (∆SN2)5 bar
b) CO2 has more entropy than dry ice at -78°C
c) Pure alumina iss crystalline solid while ruby is amorphous. And ∆Samorphous > ∆SCrystalline. So alumina has less entropy than ruby.
d) At lower pressure, entropy be higher as gas particles are far from each other. So (∆SN2)1 bar > (∆SN2)5 bar
What happens to the ions, when an ionic compound dissolves in a solvent?- a)They leave their ordered position in the crystal lattice and gets hydrated.
- b)They leave their ordered position in the crystal lattice and doesn’t hydrate.
- c)They doesn’t leave their ordered position in the crystal lattice and gets hydrated.
- d)None of the above.
Correct answer is option 'A'. Can you explain this answer?
What happens to the ions, when an ionic compound dissolves in a solvent?
a)
They leave their ordered position in the crystal lattice and gets hydrated.
b)
They leave their ordered position in the crystal lattice and doesn’t hydrate.
c)
They doesn’t leave their ordered position in the crystal lattice and gets hydrated.
d)
None of the above.
|
|
Pooja Shah answered |
When an ionic compound is dissolved in the solvent, the compound dissociate into ions and due to external medium i. e. solvent it leave its original position in crystal lattice n gets hydrated..
Eg. NaCl(aq) → Na+(aq) + Cl-(aq)
Eg. NaCl(aq) → Na+(aq) + Cl-(aq)
3 moles of a diatomic gas are heated from 127° C to 727° C at a constant pressure of 1 atm. Entropy change is (log 2.5 = 0 .4)- a)-22.98 JK-1
- b)22.98 JK-1
- c)57.4 JK-1
- d)80.42 JK-1
Correct answer is option 'D'. Can you explain this answer?
3 moles of a diatomic gas are heated from 127° C to 727° C at a constant pressure of 1 atm. Entropy change is (log 2.5 = 0 .4)
a)
-22.98 JK-1
b)
22.98 JK-1
c)
57.4 JK-1
d)
80.42 JK-1

|
Crafty Classes answered |
∆S = nCplnT2/T1 + nRlnP1/P2
Since pressure is constant, so the second term will be zero.
Or ∆S = 3×7/2×8.314×2.303×log(1000/400)
= 80.42 JK-1
Since pressure is constant, so the second term will be zero.
Or ∆S = 3×7/2×8.314×2.303×log(1000/400)
= 80.42 JK-1
The equilibrium constant for a reaction is 10. Calculate the value of ΔG° at 300K , R = R =8.314 JK−1mol−1
- a)-5.527 kJ/mol–
- b)4.744 kJ/mol
- c)-4.744 kJ/mol–
- d)- 5.744 kJ/mol
Correct answer is option 'D'. Can you explain this answer?
The equilibrium constant for a reaction is 10. Calculate the value of ΔG° at 300K , R = R =8.314 JK−1mol−1
a)
-5.527 kJ/mol–
b)
4.744 kJ/mol
c)
-4.744 kJ/mol–
d)
- 5.744 kJ/mol

|
Infinity Academy answered |
The value of equilibrium constant(k) = 10
∆G° = -2.303RTlogk
On calculating values, we will get ∆G° = -5.744kJmol-1
∆G° = -2.303RTlogk
On calculating values, we will get ∆G° = -5.744kJmol-1
In an endothermic reaction, the value of ΔH is always- a)Constant
- b)> 0
- c)< 0
- d)= 0
Correct answer is option 'C'. Can you explain this answer?
In an endothermic reaction, the value of ΔH is always
a)
Constant
b)
> 0
c)
< 0
d)
= 0
|
|
Preeti Iyer answered |
According to me, for an endothermic reaction ,the value of ∆H is always >0 .
Consider the following figure representing the increase in entropy of a substance from absolute zero to its gaseous state at some temperature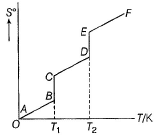 Q. ΔS° (fusion) and ΔS° (vaporisation) are respectively indicated by
Q. ΔS° (fusion) and ΔS° (vaporisation) are respectively indicated by - a)AB, BC
- b)BC,CD
- c)BC, DE
- d)CD, DE
Correct answer is option 'C'. Can you explain this answer?
Consider the following figure representing the increase in entropy of a substance from absolute zero to its gaseous state at some temperature
Q. ΔS° (fusion) and ΔS° (vaporisation) are respectively indicated by
a)
AB, BC
b)
BC,CD
c)
BC, DE
d)
CD, DE
|
|
Neha Joshi answered |
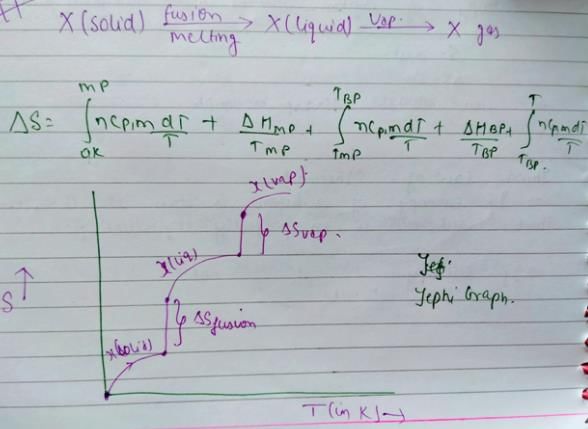
You can co-relate both graph and the result will be option c.
Standard entropies of X2, Y2 and XY3 are given below the reaction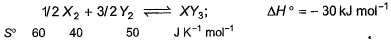 Q. At what temperature, reaction would be in equilibrium?
Q. At what temperature, reaction would be in equilibrium? - a)500 K
- b)750 K
- c)1000 K
- d)1250 K
Correct answer is option 'B'. Can you explain this answer?
Standard entropies of X2, Y2 and XY3 are given below the reaction
Q. At what temperature, reaction would be in equilibrium?
a)
500 K
b)
750 K
c)
1000 K
d)
1250 K
|
|
Suresh Reddy answered |
1/2X2 + 3/2Y2 ⟶XY3,
ΔH= −30 kJ
ΔSreaction = ∑ΔSproduct−∑ΔSreactant
X2 + 3Y2 → 2XY3
ΔH=−60 kJ
ΔSreaction = 2×50−3×40−1×60 =100−120−60=−80 JK−1mol−1
ΔG=ΔH−TΔS=0
ΔH=TΔS
1000×(−60)=−80×T
T=750 K
ΔH= −30 kJ
ΔSreaction = ∑ΔSproduct−∑ΔSreactant
X2 + 3Y2 → 2XY3
ΔH=−60 kJ
ΔSreaction = 2×50−3×40−1×60 =100−120−60=−80 JK−1mol−1
ΔG=ΔH−TΔS=0
ΔH=TΔS
1000×(−60)=−80×T
T=750 K
Among the following enthalpies, which is always less than zero?- a)ΔcH°
- b)ΔsubH°
- c)ΔmixH°
- d)ΔfH°
Correct answer is option 'A'. Can you explain this answer?
Among the following enthalpies, which is always less than zero?
a)
ΔcH°
b)
ΔsubH°
c)
ΔmixH°
d)
ΔfH°
|
|
Neha Joshi answered |
The symbol represents enthalpy of combustion which is always exothermic. For exothermic rxn delta H is always -ve.
For the process,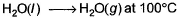 and 1 atmosphere pressure, the correct choice is[JEE Advanced 2014]
and 1 atmosphere pressure, the correct choice is[JEE Advanced 2014]- a)ΔS(System) > 0, ΔS(surrounding) > 0
- b)ΔS(System) > 0, ΔS(surrounding) < 0
- c)ΔS(System) < 0, ΔS(surrounding) > 0
- d)ΔS(System) < 0, ΔS(surrounding) < 0
Correct answer is option 'B'. Can you explain this answer?
For the process,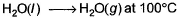 and 1 atmosphere pressure, the correct choice is
and 1 atmosphere pressure, the correct choice is
[JEE Advanced 2014]
a)
ΔS(System) > 0, ΔS(surrounding) > 0
b)
ΔS(System) > 0, ΔS(surrounding) < 0
c)
ΔS(System) < 0, ΔS(surrounding) > 0
d)
ΔS(System) < 0, ΔS(surrounding) < 0
|
|
Lavanya Menon answered |
At 100°C and 1 atmosphere pressure H2O (l) ⇋ H2O(g) is at equilibrium. For equilibrium


The difference between Cp and Cv is equal to- a)8.314 ergs K-1 mol-1
- b)2 kcal mol-1
- c)8.314 JK-1mol-1
- d)1.99 cal
Correct answer is option 'C'. Can you explain this answer?
The difference between Cp and Cv is equal to
a)
8.314 ergs K-1 mol-1
b)
2 kcal mol-1
c)
8.314 JK-1mol-1
d)
1.99 cal
|
|
Om Desai answered |
Cp-Cv = R
R is gas constant whose value is 8.314 J/K/mol
R is gas constant whose value is 8.314 J/K/mol
GivenI. C (diamond) + O2(g) → CO2(g) ; ΔH° = - 91.0 kcdl mol-1
II. C(graphite) + O2(g) → CO2(g) ; ΔH° = - 94.0 kcal mol-1Q. At 298 K, 2.4 kg of carbon (diamond) is converted into graphite form. Thus, entropy change is- a)10.07 cal K-1
- b)2.013 kcal K-1
- c)305.4 cal K-1
- d)- 2.013 kcal K-1
Correct answer is option 'B'. Can you explain this answer?
Given
I. C (diamond) + O2(g) → CO2(g) ; ΔH° = - 91.0 kcdl mol-1
II. C(graphite) + O2(g) → CO2(g) ; ΔH° = - 94.0 kcal mol-1
II. C(graphite) + O2(g) → CO2(g) ; ΔH° = - 94.0 kcal mol-1
Q. At 298 K, 2.4 kg of carbon (diamond) is converted into graphite form. Thus, entropy change is
a)
10.07 cal K-1
b)
2.013 kcal K-1
c)
305.4 cal K-1
d)
- 2.013 kcal K-1
|
|
Gaurav Kumar answered |
The reaction is
C(diamond) → C(graphite) ∆H = (94-91) = 3 kcal mol-1
∆S = ∆H/T
∆H = (94-91)×2.4×103/12
= 600 kcal
∆S = 600/298 = 2.013 kcal K-1
C(diamond) → C(graphite) ∆H = (94-91) = 3 kcal mol-1
∆S = ∆H/T
∆H = (94-91)×2.4×103/12
= 600 kcal
∆S = 600/298 = 2.013 kcal K-1
Which reaction, with the following values of ΔH and ΔS at 400 K is spontaneous and endothermic?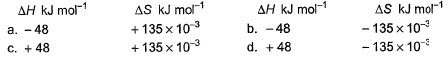
- a)a
- b)b
- c)c
- d)d
Correct answer is option 'C'. Can you explain this answer?
Which reaction, with the following values of ΔH and ΔS at 400 K is spontaneous and endothermic?
a)
a
b)
b
c)
c
d)
d
|
|
Om Desai answered |
∆G = ∆H - T∆S
For opt (c), ∆G = 48000 - 400(135)
= 48000 - 54000
= -6000
∆G is -ve
Therefore reaction is spontaneous.
For opt (c), ∆G = 48000 - 400(135)
= 48000 - 54000
= -6000
∆G is -ve
Therefore reaction is spontaneous.
Direction (Q. Nos. 1-10) This section contains 10 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.Q. Entropy change for the following reversible process is 1 mole H2O(l, 1 atm, 100°C ) 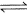 1 mole H2O (g , 1 atm, 100°C)(ΔHvap = 40850 J mol-1)
1 mole H2O (g , 1 atm, 100°C)(ΔHvap = 40850 J mol-1)- a)+109.52 JK-1 mol-1
- b)-109.52 JK-1 mol-1
- c)0.00 JK-1 mol-1
- d)+6.084 JK-1 mol-1
Correct answer is option 'A'. Can you explain this answer?
Direction (Q. Nos. 1-10) This section contains 10 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Q. Entropy change for the following reversible process is 1 mole H2O
(l, 1 atm, 100°C ) 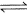 1 mole H2O (g , 1 atm, 100°C)(ΔHvap = 40850 J mol-1)
1 mole H2O (g , 1 atm, 100°C)(ΔHvap = 40850 J mol-1)
a)
+109.52 JK-1 mol-1
b)
-109.52 JK-1 mol-1
c)
0.00 JK-1 mol-1
d)
+6.084 JK-1 mol-1
|
|
Krishna Iyer answered |
∆S=Change in energy/Absolute temp =∆H/(100+273)K =40850/373 = 109.52 J/K/mol
The enthalpies of fusion for all reactions are- a)Negative
- b)Depends on the type of substance
- c)Zero
- d)Positive
Correct answer is option 'D'. Can you explain this answer?
The enthalpies of fusion for all reactions are
a)
Negative
b)
Depends on the type of substance
c)
Zero
d)
Positive
|
|
Lavanya Menon answered |
The enthalpies of fusion for all reactions are always positive because melting of a solid is an endothermic process in which heat is supplied to the reaction Hence the sum of enthalpies of the product is greater than the sum of enthalpies of the reactant.
Given,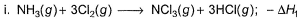
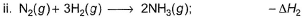
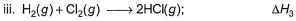
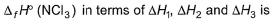
- a)
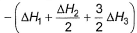
- b)
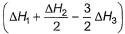
- c)
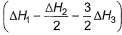
- d)
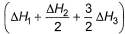
Correct answer is option 'A'. Can you explain this answer?
Given,
a)
b)
c)
d)
|
|
Gaurav Kumar answered |
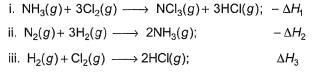
Applying i-ii/2-3/2iii
We get ∆fH of NCl3 in terms of ∆H1, ∆H2 and ∆H3
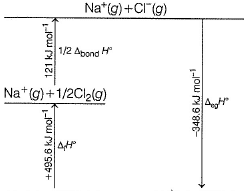
Following diagram represents Born-Haber cycle to determine lattice energy of NaCI(s). It is based on Hess’s law of constant heat summation. ΔlatticeH° of 
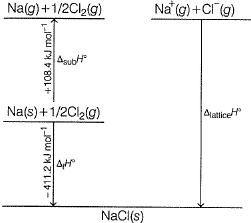
- a)-788 kJ mol-1
- b)+788 kJ mol-1
- c)-411.2 kJ mol-1
- d)+411.2 kJ mol-1
Correct answer is option 'A'. Can you explain this answer?
Following diagram represents Born-Haber cycle to determine lattice energy of NaCI(s). It is based on Hess’s law of constant heat summation. ΔlatticeH° of
a)
-788 kJ mol-1
b)
+788 kJ mol-1
c)
-411.2 kJ mol-1
d)
+411.2 kJ mol-1

|
Meghana Devi answered |
-411.2 - 108.4 - 495.6 - 121.0 + 348.6=-787.6~-788
Direction (Q. Nos. 12-15) This section contains 4 questions. when worked out will result in an integer from 0 to 9 (both inclusive).Q. Based on (BE) values, ΔrH° of the following reaction 298 K is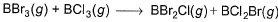
Correct answer is '0'. Can you explain this answer?
Direction (Q. Nos. 12-15) This section contains 4 questions. when worked out will result in an integer from 0 to 9 (both inclusive).
Q. Based on (BE) values, ΔrH° of the following reaction 298 K is
|
|
Krishna Iyer answered |
Let the BE value of B---Br bond be x and B---Cl bond be y
BE of BBr3 is 3x, BCl3=3y , BBr2Cl=2x+y ,BCl2Br=2y+x
then ∆Hr=Hr(products)-Hr(reactants)=(2x+y+2y+x) - (3x+3y)=0
BE of BBr3 is 3x, BCl3=3y , BBr2Cl=2x+y ,BCl2Br=2y+x
then ∆Hr=Hr(products)-Hr(reactants)=(2x+y+2y+x) - (3x+3y)=0
Given, HCI (g) → H (g) + Cl (g) at 298 K (say temperature T K),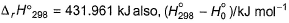 For
For Q. Thus, for the given reaction
Q. Thus, for the given reaction 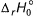 (at 0 K) is
(at 0 K) is- a)428.136 kJ
- b)-428.136 kJ
- c)431.961 kJ
- d)-431.961 kJ
Correct answer is option 'A'. Can you explain this answer?
Given, HCI (g) → H (g) + Cl (g) at 298 K (say temperature T K),
For
Q. Thus, for the given reaction 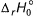 (at 0 K) is
(at 0 K) is
a)
428.136 kJ
b)
-428.136 kJ
c)
431.961 kJ
d)
-431.961 kJ

|
Infinity Academy answered |
Ho= Ho of product - Ho of reactant
so ho =(6.197 + 6.272 ) - 8.644
= 3.825
Ho298-Ho = 431.961 - 3.825=428.136532873
so ho =(6.197 + 6.272 ) - 8.644
= 3.825
Ho298-Ho = 431.961 - 3.825=428.136532873
What is the value of γ for a monoatomic gas?- a)1.40
- b)1.30
- c)1.67
- d)1.20
Correct answer is option 'C'. Can you explain this answer?
What is the value of γ for a monoatomic gas?
a)
1.40
b)
1.30
c)
1.67
d)
1.20
|
|
Hansa Sharma answered |
For monatomic species, γ = 1.67
Passage II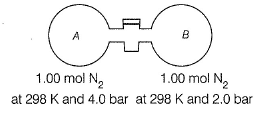 The stopcock connecting A and B is of negligible volume. Stopcock is opened and gases are allowed to mix isothermally. Q. Final pressure set up is
The stopcock connecting A and B is of negligible volume. Stopcock is opened and gases are allowed to mix isothermally. Q. Final pressure set up is- a)6.00 bar
- b)4.00 bar
- c)2.00 bar
- d)2.67 bar
Correct answer is option 'D'. Can you explain this answer?
Passage II
The stopcock connecting A and B is of negligible volume. Stopcock is opened and gases are allowed to mix isothermally.
Q. Final pressure set up is
a)
6.00 bar
b)
4.00 bar
c)
2.00 bar
d)
2.67 bar

|
Lohit Matani answered |
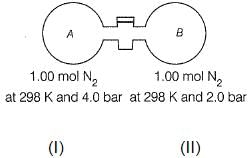
Volume in (I) = nRT/P1 = 1× 0.0821× 298/4 = 6.11 L
Volume in (II) = nRT/P2 = 1× 0.0821× 298/2 = 12.23 L
Total volume = 18.34 L
Applying PV = nRT at final condition,
P = 2× 0.0821× 298/18.34 = 2.66
Which of the following statements is correct about the process of evaporation of water from an open beaker?- a)It is a spontaneous and endothermic process.
- b)It is a non-spontaneous and endothermic process.
- c)It is a spontaneous and exothermic process.
- d)It is a non-spontaneous and exothermic process.
Correct answer is option 'A'. Can you explain this answer?
Which of the following statements is correct about the process of evaporation of water from an open beaker?
a)
It is a spontaneous and endothermic process.
b)
It is a non-spontaneous and endothermic process.
c)
It is a spontaneous and exothermic process.
d)
It is a non-spontaneous and exothermic process.
|
|
Geetika Shah answered |
Evaporation of water from an open pot is a spontaneous process. As it starts on its own without any external agent. However it takes heat from surrounding means that it is endothermic.
The value of log10 K for a reaction, A  B is (Given, ΔH°298 = - 54.07 kJ mol-1;ΔS°298 = + 10 JK-1 mol-1; R = 8.314 JK-1 mol-1 2.303 x 8.314 x 298 = 5705) [IITJEE2007]
B is (Given, ΔH°298 = - 54.07 kJ mol-1;ΔS°298 = + 10 JK-1 mol-1; R = 8.314 JK-1 mol-1 2.303 x 8.314 x 298 = 5705) [IITJEE2007]- a)5
- b)10
- c)95
- d)100
Correct answer is option 'B'. Can you explain this answer?
The value of log10 K for a reaction, A  B is (Given, ΔH°298 = - 54.07 kJ mol-1;
B is (Given, ΔH°298 = - 54.07 kJ mol-1;
ΔS°298 = + 10 JK-1 mol-1; R = 8.314 JK-1 mol-1 2.303 x 8.314 x 298 = 5705)
[IITJEE2007]
a)
5
b)
10
c)
95
d)
100
|
|
Lavanya Menon answered |
∆G° = ∆H° - T∆S°
= -54070 - 298 10
= -57050
∆G° = -2.303 RT log10k
-57050 = -2.303 8.314 298 log10k
57050 = 5705 log10k
log10k = 10
= -54070 - 298 10
= -57050
∆G° = -2.303 RT log10k
-57050 = -2.303 8.314 298 log10k
57050 = 5705 log10k
log10k = 10
Direction (Q. Nos. 1-7) This section contains 7 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.Given,The enthalpies of elements in their standard states are taken as zero. The enthalpy of formation of a compound- a)is always negative
- b)is always positive
- c)may be positive or negative
- d)is never negative
Correct answer is option 'C'. Can you explain this answer?
Direction (Q. Nos. 1-7) This section contains 7 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Given,
The enthalpies of elements in their standard states are taken as zero. The enthalpy of formation of a compound
a)
is always negative
b)
is always positive
c)
may be positive or negative
d)
is never negative
|
|
Om Desai answered |
Depend on whether the formation of compounds is exothermic or endothermic.For exothermic reaction, enthalpy of formation would be negative and for endothermic,it is positive.
Direction (Q. Nos. 9 and 10) This section contains a paragraph, wach describing theory, experiments, data etc. three Questions related to paragraph have been given.Each question have only one correct answer among the four given ptions (a),(b),(c),(d).Consider the following thermochemical equations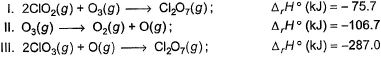
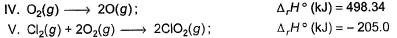
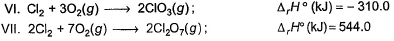 Q. ΔrH° of the following thermochemical reaction isCIO2 (g) + O (g) → CIO3 (g)
Q. ΔrH° of the following thermochemical reaction isCIO2 (g) + O (g) → CIO3 (g)- a)-197 kJ
- b)+197 kJ
- c)+312 kJ
- d)+212 kJ
Correct answer is option 'A'. Can you explain this answer?
Direction (Q. Nos. 9 and 10) This section contains a paragraph, wach describing theory, experiments, data etc. three Questions related to paragraph have been given.Each question have only one correct answer among the four given ptions (a),(b),(c),(d).
Consider the following thermochemical equations
Q. ΔrH° of the following thermochemical reaction is
CIO2 (g) + O (g) → CIO3 (g)
a)
-197 kJ
b)
+197 kJ
c)
+312 kJ
d)
+212 kJ
|
|
Geetika Shah answered |
The question can be simply done by understanding that this reaction is endothermic so ∆H is negative.
Hence A is correct.
The solubility of fluorides is much less as compared to corresponding chlorides- a)because of low lattice enthalpy.
- b)because of high lattice enthalpy.
- c)because of low ionization energy.
- d)because of high ionization energy.
Correct answer is option 'B'. Can you explain this answer?
The solubility of fluorides is much less as compared to corresponding chlorides
a)
because of low lattice enthalpy.
b)
because of high lattice enthalpy.
c)
because of low ionization energy.
d)
because of high ionization energy.
|
|
Om Desai answered |
Due to high lattice enthalpy of fluorides, it is difficult to break the lattice of fluoride compounds(due to the small size of fluorine, so the lattice is strong). SO the solubility of fluorides is less than chlorides.
Heat of combustion of CH2CO(g) is - 981.1 kJ mol-1 and that of CH4 (g) is - 802.3 kJat 298 K. If 1247.0 kJ of heat is released in the following change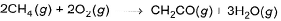 Q. How many moles of CH4(g)are used?
Q. How many moles of CH4(g)are used?
Correct answer is '4'. Can you explain this answer?
Heat of combustion of CH2CO(g) is - 981.1 kJ mol-1 and that of CH4 (g) is - 802.3 kJat 298 K. If 1247.0 kJ of heat is released in the following change
Q. How many moles of CH4(g)are used?
|
|
Pooja Shah answered |
[2CH4 + 2O2 → CH2O + H2O]n
∆HC = n[-981.1-(-8023×2)]
1247 = n[-981.1+1604.6]
N[623.5] = 1247
N = 4
So, no of moles of CH4 = n×2 = 2×2 = 4
∆HC = n[-981.1-(-8023×2)]
1247 = n[-981.1+1604.6]
N[623.5] = 1247
N = 4
So, no of moles of CH4 = n×2 = 2×2 = 4
10 dm3 of an ideal monoatomic gas at 27° C and 1.01 x 105 Nm-2 pressure are heated at constant pressure to 127°C. Thus entropy change is- a)2.422 JK-1
- b)5.98 JK-1
- c)- 2.422 JK-1
- d)- 5.981 JK-1
Correct answer is option 'A'. Can you explain this answer?
10 dm3 of an ideal monoatomic gas at 27° C and 1.01 x 105 Nm-2 pressure are heated at constant pressure to 127°C. Thus entropy change is
a)
2.422 JK-1
b)
5.98 JK-1
c)
- 2.422 JK-1
d)
- 5.981 JK-1
|
|
Gaurav Kumar answered |
For isobaric process, we have ∆S =nCpln(T2/T1)
T2 = 273+127 = 400K and T1 = 273+227 = 300K
Applying pV = nRT at initial condition,
1×10 = n×0.0821×300
n = 0.40
Applying ∆S =nCpln(T2/T1)
∆S =0.40×5/2R×ln(400/300) = 2.38 JK-1
T2 = 273+127 = 400K and T1 = 273+227 = 300K
Applying pV = nRT at initial condition,
1×10 = n×0.0821×300
n = 0.40
Applying ∆S =nCpln(T2/T1)
∆S =0.40×5/2R×ln(400/300) = 2.38 JK-1
ΔHvap = 30 kJ mol-1 and ΔSvap = 75 J mol-1 K-1. Thus, temperature of the vapour at 1 atm is[IIT JEE 2004]- a)400 K
- b)350 K
- c)298 K
- d)250 K
Correct answer is option 'A'. Can you explain this answer?
ΔHvap = 30 kJ mol-1 and ΔSvap = 75 J mol-1 K-1. Thus, temperature of the vapour at 1 atm is
[IIT JEE 2004]
a)
400 K
b)
350 K
c)
298 K
d)
250 K
|
|
Gaurav Kumar answered |
Vapour pressure is equal to atmospheric pressure ,it means the substance is at boiling point
At boiling point, liquid and Gas are in equilibrium. Therefore dG=0
dG = H - TdS
dG = 0
⇒H = TdS
⇒T = H/dS
⇒ T = 30 103/75 = 400K
At boiling point, liquid and Gas are in equilibrium. Therefore dG=0
dG = H - TdS
dG = 0
⇒H = TdS
⇒T = H/dS
⇒ T = 30 103/75 = 400K
Passage IIFor oxidation of iron at 298 K, 4 Fe (s) + 3 O2(g) → 2 Fe2O3(s)
ΔS° = - 549.4 JK-1 mol-1 and ΔH ° = - 1648 . 0 kJ mol-1 Q. Select the correct alternate.- a)This reaction is spontaneous
- b)ΔS° (surrounding) > 0
- c)ΔS° (total) > 0
- d)All of these are correct statements
Correct answer is option 'A'. Can you explain this answer?
Passage II
For oxidation of iron at 298 K, 4 Fe (s) + 3 O2(g) → 2 Fe2O3(s)
ΔS° = - 549.4 JK-1 mol-1 and ΔH ° = - 1648 . 0 kJ mol-1
ΔS° = - 549.4 JK-1 mol-1 and ΔH ° = - 1648 . 0 kJ mol-1
Q. Select the correct alternate.
a)
This reaction is spontaneous
b)
ΔS° (surrounding) > 0
c)
ΔS° (total) > 0
d)
All of these are correct statements

|
Infinity Academy answered |
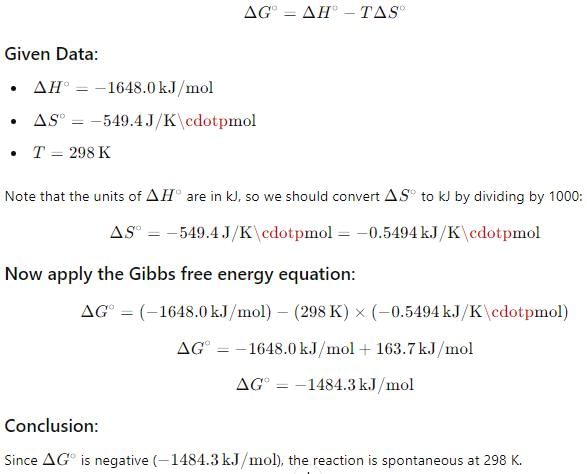
Thus, the correct alternate would indicate that the oxidation of iron is spontaneous at 298 K.
4o
On which of the following factors the enthalpy change of a reaction is NOT dependent?- a)Nature of reactants and products
- b)Various intermediate reactions
- c)State of reactants and products
- d)Enthalpy of reactants and products
Correct answer is option 'B'. Can you explain this answer?
On which of the following factors the enthalpy change of a reaction is NOT dependent?
a)
Nature of reactants and products
b)
Various intermediate reactions
c)
State of reactants and products
d)
Enthalpy of reactants and products
|
|
Nandini Iyer answered |
The enthalpy change for a reaction does not depend upon the. use of different reactants for the same product. difference in initial or final temperatures of involved substances.
The increasing order of enthalpy of vaporization of NH3, PH3, and AsH3 is- a)NH3, AsH3, PH3
- b)NH3, PH3, AsH3
- c)AsH3, PH3, NH3
- d)PH3, AsH3, NH3
Correct answer is option 'D'. Can you explain this answer?
The increasing order of enthalpy of vaporization of NH3, PH3, and AsH3 is
a)
NH3, AsH3, PH3
b)
NH3, PH3, AsH3
c)
AsH3, PH3, NH3
d)
PH3, AsH3, NH3
|
|
Preeti Iyer answered |
The inter-particle forces are strongest in NH3 (Hydrogen bonds). In PH3 and AsH3 the inter-particle forces are dipole-dipole forces which are relatively stronger in AsH3. So the increasing order of vaporization is PH3, AsH3, and NH3 .
Entropy change when 2 moles of an ideal gas expands reversibly from an initial volume of 1 dm3 to a final volume of 10 dm3 at a constant temperature of 298 K is- a)5705.8 JK-1
- b)19.15 JK-1
- c)38.29 JK-1
- d)-19.15 JK-1
Correct answer is option 'C'. Can you explain this answer?
Entropy change when 2 moles of an ideal gas expands reversibly from an initial volume of 1 dm3 to a final volume of 10 dm3 at a constant temperature of 298 K is
a)
5705.8 JK-1
b)
19.15 JK-1
c)
38.29 JK-1
d)
-19.15 JK-1
|
|
Om Desai answered |
∆S= 2.303nR × log(V2/V1)Here n=2, R=8.314, V2= 10, V1= 1
The measured enthalpy change for burning of ketene (g) (CH2CO) is - 981.1 kJ mo-1 at 298 K ,CH2CO (g)+ 2O2(g) → 2CO2 (g) + H2O (g) and that of CH4 (g) is - 802.3 kJ mol-1 at 298 K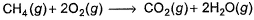 Thus, enthalpy change at 298 K for the following thermochemical reaction is
Thus, enthalpy change at 298 K for the following thermochemical reaction is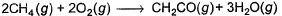
- a)-623.5 kJ
- b)+623.5 kJ
- c)-178.8 kJ
- d)+802.3 kJ
Correct answer is option 'A'. Can you explain this answer?
The measured enthalpy change for burning of ketene (g) (CH2CO) is - 981.1 kJ mo-1 at 298 K ,CH2CO (g)+ 2O2(g) → 2CO2 (g) + H2O (g) and that of CH4 (g) is - 802.3 kJ mol-1 at 298 K
Thus, enthalpy change at 298 K for the following thermochemical reaction is
a)
-623.5 kJ
b)
+623.5 kJ
c)
-178.8 kJ
d)
+802.3 kJ
|
|
Rajesh Gupta answered |
CH2CO (g)+ 2O2(g) → 2CO2 (g) + H2O (g) -(i)
CH4 (g)+ 2O2(g) → CO2 (g) + 2H2O (g) -(ii)
CH4 (g)+ 2O2(g) → CO2 (g) + 2H2O (g) -(ii)
On 2(ii)-(i)
2CH4 (g)+ 2O2(g) → CH2CO (g) + 3H2O (g) -(iii)
∆H(iii) = 2∆H(ii) - ∆H(i) = [2 (-802.3) -(-981.1)] -623.5 kJ mol-1
∆H(iii) = 2∆H(ii) - ∆H(i) = [2 (-802.3) -(-981.1)] -623.5 kJ mol-1
Consider the following reactions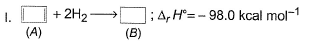
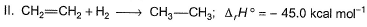 Q. What is the resonance energy (in kcal) of A?
Q. What is the resonance energy (in kcal) of A?
Correct answer is '8'. Can you explain this answer?
Consider the following reactions
Q. What is the resonance energy (in kcal) of A?
|
|
Gaurav Kumar answered |
Resonance energy = (Observed ∆H) - (Theoretical ∆H)
= (-98.0) - 2(-45.0)
= -8 kcal mol-1
So we have Resnance energy = 8
= (-98.0) - 2(-45.0)
= -8 kcal mol-1
So we have Resnance energy = 8
The condition in which q will be equal to ΔH of a system is- a)constant P and T
- b)constant P and V
- c)constant P, T and V
- d)constant T and V
Correct answer is option 'A'. Can you explain this answer?
The condition in which q will be equal to ΔH of a system is
a)
constant P and T
b)
constant P and V
c)
constant P, T and V
d)
constant T and V
|
|
Preeti Iyer answered |
Under the condition, the heat q of the reaction is equal to the enthalpy change ΔH of the system. Under constant pressure and temperature, the free energy in a reaction is known as Gibbs free energy G.
G = H - TS
G = H - TS
The chemical reaction:
CH4 (g) + 2O2 (g) → CO2 (g) + 2H2O (l) ; Δh= -890.4kJ is- a)endothermic
- b)exothermic
- c)unpredictable
- d)depend on the reaction conditions
Correct answer is option 'B'. Can you explain this answer?
The chemical reaction:
CH4 (g) + 2O2 (g) → CO2 (g) + 2H2O (l) ; Δh= -890.4kJ is
CH4 (g) + 2O2 (g) → CO2 (g) + 2H2O (l) ; Δh= -890.4kJ is
a)
endothermic
b)
exothermic
c)
unpredictable
d)
depend on the reaction conditions
|
|
Preethi Chakraborty answered |
Since heat is released as shown in the reaction with a negative sign, the above reaction is exothermic.
For a spontaneous chemical process, the free energy change is- a)not measurable
- b)negative
- c)positive
- d)zero
Correct answer is option 'B'. Can you explain this answer?
For a spontaneous chemical process, the free energy change is
a)
not measurable
b)
negative
c)
positive
d)
zero

|
Jatin Dasgupta answered |
Understanding Free Energy Change in Chemical Processes
In spontaneous chemical processes, the change in free energy is a crucial indicator of whether a reaction can occur without external intervention.
What is Free Energy?
- Free energy, often represented as G, is a thermodynamic quantity that combines enthalpy and entropy.
- It helps predict the direction of chemical reactions.
Spontaneous Processes
- A spontaneous process is one that occurs naturally under specific conditions without needing to be driven by an external force.
- For such processes, the system tends to move towards equilibrium.
Free Energy Change (ΔG)
- The change in free energy (ΔG) is calculated as the difference in free energy between the products and reactants.
- The sign of ΔG determines the spontaneity of the reaction.
Negative Free Energy Change
- If ΔG is negative (ΔG < 0),="" it="" indicates="" that="" the="" reaction="" can="" occur="" />
- A negative value signifies that the products have lower free energy compared to the reactants, meaning energy is released during the process.
Implications of ΔG
- A negative ΔG implies that the reaction is thermodynamically favorable.
- This release of energy often results in increased entropy, a natural tendency for systems to achieve greater disorder.
Conclusion
- Therefore, for spontaneous chemical processes, the free energy change is indeed negative, confirming that option 'B' is correct.
- Understanding this concept is vital in predicting reaction behavior in chemistry.
In spontaneous chemical processes, the change in free energy is a crucial indicator of whether a reaction can occur without external intervention.
What is Free Energy?
- Free energy, often represented as G, is a thermodynamic quantity that combines enthalpy and entropy.
- It helps predict the direction of chemical reactions.
Spontaneous Processes
- A spontaneous process is one that occurs naturally under specific conditions without needing to be driven by an external force.
- For such processes, the system tends to move towards equilibrium.
Free Energy Change (ΔG)
- The change in free energy (ΔG) is calculated as the difference in free energy between the products and reactants.
- The sign of ΔG determines the spontaneity of the reaction.
Negative Free Energy Change
- If ΔG is negative (ΔG < 0),="" it="" indicates="" that="" the="" reaction="" can="" occur="" />
- A negative value signifies that the products have lower free energy compared to the reactants, meaning energy is released during the process.
Implications of ΔG
- A negative ΔG implies that the reaction is thermodynamically favorable.
- This release of energy often results in increased entropy, a natural tendency for systems to achieve greater disorder.
Conclusion
- Therefore, for spontaneous chemical processes, the free energy change is indeed negative, confirming that option 'B' is correct.
- Understanding this concept is vital in predicting reaction behavior in chemistry.
The condition for free expansion of an ideal gas under adiabatic condition is
- a)q = 0, w = 0, ΔT = 0
- b)q ≠ 0, w = 0, ΔT = 0
- c)q = 0, w = 0, ΔT ≠ 0
- d)q = 0, w > 0, ΔT = 0
Correct answer is option 'A'. Can you explain this answer?
The condition for free expansion of an ideal gas under adiabatic condition is
a)
q = 0, w = 0, ΔT = 0
b)
q ≠ 0, w = 0, ΔT = 0
c)
q = 0, w = 0, ΔT ≠ 0
d)
q = 0, w > 0, ΔT = 0

|
EduRev NEET answered |
Explanation:
For free expansion of ideal gas,
Pext = 0
WpV = 0
For adiabatic process,
q = 0
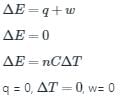
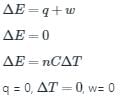
Conclusion:
Thus, the condition for free expansion of an ideal gas under adiabatic condition is q = 0, w = 0, ΔT = 0.
Which of the following is true about a spontaneous process?- a)Spontaneity does not depend on Gibbs energy.
- b)Gibbs energy change is less than 0.
- c)Gibbs energy change is 0.
- d)Gibbs energy change is greater than 0.
Correct answer is option 'B'. Can you explain this answer?
Which of the following is true about a spontaneous process?
a)
Spontaneity does not depend on Gibbs energy.
b)
Gibbs energy change is less than 0.
c)
Gibbs energy change is 0.
d)
Gibbs energy change is greater than 0.

|
Ameya Basu answered |
A spontaneous process is the time-evolution of a system in which it releases free energy and it moves to a lower, more thermodynamically stable energy state. For cases involving an isolated system where no energy is exchanged with the surroundings, spontaneous processes are characterized by an increase in entropy
Which of the following reactions is an endothermic process?- a)C + O2 → CO2 ; ΔH = -x kj
- b)2H2 + O2 → 2H2O ; ΔH = -y kj
- c)CH4 + 2O2 → CO2 + 2H2O ; ΔH = -q kj
- d)N2 + O2 → 2NO ; ΔH = z kj
Correct answer is option 'D'. Can you explain this answer?
Which of the following reactions is an endothermic process?
a)
C + O2 → CO2 ; ΔH = -x kj
b)
2H2 + O2 → 2H2O ; ΔH = -y kj
c)
CH4 + 2O2 → CO2 + 2H2O ; ΔH = -q kj
d)
N2 + O2 → 2NO ; ΔH = z kj
|
|
Megha Desai answered |
For endothermic reactions, enthalpy is with no-ve sign in the end of reaction.
Which of the following is extensive property?- a)Specific heat capacity
- b)Entropy
- c)Temperature
- d)Refractive index
Correct answer is option 'B'. Can you explain this answer?
Which of the following is extensive property?
a)
Specific heat capacity
b)
Entropy
c)
Temperature
d)
Refractive index
|
|
Tanishq Unni answered |
Extensive Property:
An extensive property is a physical property of a system that depends on the amount of substance present in the system. In other words, extensive properties are additive and scale with the size or amount of the system. The value of an extensive property changes when the size or amount of the system changes.
Explanation:
Out of the given options, the correct answer is option 'B' - Entropy.
Entropy is a measure of the disorder or randomness of a system. It is a thermodynamic property that quantifies the number of microscopic configurations that a system can have. Entropy is an extensive property because it depends on the size or amount of the system.
Comparison with Other Options:
a) Specific Heat Capacity:
Specific heat capacity is the amount of heat energy required to raise the temperature of a substance by a certain amount. It is an intensive property because it does not depend on the amount of substance present. The specific heat capacity of a substance remains the same regardless of the size or amount of the substance.
b) Entropy:
As mentioned earlier, entropy is an extensive property as it depends on the amount of substance present in the system. If the system size or amount changes, the entropy of the system will also change proportionally.
c) Temperature:
Temperature is an intensive property because it does not depend on the amount of substance present. The temperature of a substance remains the same regardless of the size or amount of the substance.
d) Refractive Index:
Refractive index is a measure of how light propagates through a medium. It is an intensive property because it does not depend on the amount of substance present. The refractive index of a substance remains the same regardless of the size or amount of the substance.
Conclusion:
Among the given options, entropy is the only extensive property. It is a thermodynamic property that depends on the amount of substance present in the system. The other options, specific heat capacity, temperature, and refractive index, are intensive properties that do not depend on the amount of substance present.
An extensive property is a physical property of a system that depends on the amount of substance present in the system. In other words, extensive properties are additive and scale with the size or amount of the system. The value of an extensive property changes when the size or amount of the system changes.
Explanation:
Out of the given options, the correct answer is option 'B' - Entropy.
Entropy is a measure of the disorder or randomness of a system. It is a thermodynamic property that quantifies the number of microscopic configurations that a system can have. Entropy is an extensive property because it depends on the size or amount of the system.
Comparison with Other Options:
a) Specific Heat Capacity:
Specific heat capacity is the amount of heat energy required to raise the temperature of a substance by a certain amount. It is an intensive property because it does not depend on the amount of substance present. The specific heat capacity of a substance remains the same regardless of the size or amount of the substance.
b) Entropy:
As mentioned earlier, entropy is an extensive property as it depends on the amount of substance present in the system. If the system size or amount changes, the entropy of the system will also change proportionally.
c) Temperature:
Temperature is an intensive property because it does not depend on the amount of substance present. The temperature of a substance remains the same regardless of the size or amount of the substance.
d) Refractive Index:
Refractive index is a measure of how light propagates through a medium. It is an intensive property because it does not depend on the amount of substance present. The refractive index of a substance remains the same regardless of the size or amount of the substance.
Conclusion:
Among the given options, entropy is the only extensive property. It is a thermodynamic property that depends on the amount of substance present in the system. The other options, specific heat capacity, temperature, and refractive index, are intensive properties that do not depend on the amount of substance present.
Direction (Q. Nos. 19 and 20) This section contains 2 questions. Each question, when worked out will result in an integer from 0 fo 9 (both inclusive).Q. Consider two Carnot engines (1) and (2)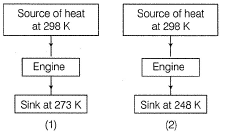 Efficiency η2
Efficiency η2
Derive the value of η2 η1
Correct answer is '2'. Can you explain this answer?
Direction (Q. Nos. 19 and 20) This section contains 2 questions. Each question, when worked out will result in an integer from 0 fo 9 (both inclusive).
Q. Consider two Carnot engines (1) and (2)
Efficiency η2
Derive the value of η2 η1
Derive the value of η2 η1
|
|
Om Desai answered |
η1 = 1-273/298 = 25/298
η2 = 1-248/298 = 50/298
η2/η1 = (50/298)/25/298 = 2
η2 = 1-248/298 = 50/298
η2/η1 = (50/298)/25/298 = 2
The value of ΔS for spontaneous process is- a)ΔSTotal is constant
- b)ΔSTotal > 0
- c)ΔSTotal < 0
- d)ΔSTotal = 0
Correct answer is option 'B'. Can you explain this answer?
The value of ΔS for spontaneous process is
a)
ΔSTotal is constant
b)
ΔSTotal > 0
c)
ΔSTotal < 0
d)
ΔSTotal = 0

|
Prisha Yadav answered |
Something is subjective and can vary depending on individual perspectives and circumstances. It can be determined based on various factors such as usefulness, scarcity, demand, and personal preferences.
Direction (Q. Nos. 11) Choice the correct combination of elements and column I and coloumn II are given as option (a), (b), (c) and (d), out of which ONE option is correct.Q. For liquid water at 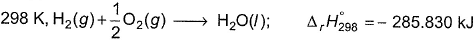
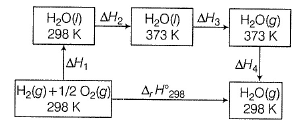
 Compare the parameters ΔH1, ΔH2 ... in Column I with the corresponding values in kJ in Column II.
Compare the parameters ΔH1, ΔH2 ... in Column I with the corresponding values in kJ in Column II.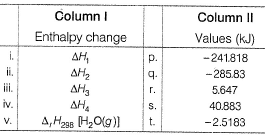

- a)a
- b)b
- c)c
- d)d
Correct answer is option 'D'. Can you explain this answer?
Direction (Q. Nos. 11) Choice the correct combination of elements and column I and coloumn II are given as option (a), (b), (c) and (d), out of which ONE option is correct.
Q. For liquid water at
Compare the parameters ΔH1, ΔH2 ... in Column I with the corresponding values in kJ in Column II.
a)
a
b)
b
c)
c
d)
d
|
|
Pooja Shah answered |
∆H1=∆rH298=-285.83kJmol-¹
∆H²=Cp(liq)∆T=75.29×75 =5.64kJmol-¹
∆H³=∆Hvap =40.88kJmol-¹
∆H⁴=Cp(gas)∆T=35.57×(-75) =-2.5183kJmol-¹
∆H²=Cp(liq)∆T=75.29×75 =5.64kJmol-¹
∆H³=∆Hvap =40.88kJmol-¹
∆H⁴=Cp(gas)∆T=35.57×(-75) =-2.5183kJmol-¹
What is the change in the entropy of water, When ice melts into water?- a)Entropy of water increases.
- b)Entropy of water becomes zero.
- c)Entropy of water decreases.
- d)Entropy of water remains same.
Correct answer is option 'A'. Can you explain this answer?
What is the change in the entropy of water, When ice melts into water?
a)
Entropy of water increases.
b)
Entropy of water becomes zero.
c)
Entropy of water decreases.
d)
Entropy of water remains same.

|
Raghav Yadav answered |
The greater the randomness in a system, greater is its entropy. The randomness is greater in liquid state as compared to solid state so the entropy increases when ice melts into water.
Direction (Q. No. 9) This sectionis based on statement I and Statement II. Select the correct answer from the code given below.Q. Statement I :Every endothermic reaction is spontaneous if TΔS > ΔH.Statement II : Sign of ΔG is the true criterion for deciding spontaneity of a reaction.- a)Both Statement I and Statement II are correct and Statement II is the correct explanation of Statement I
- b)Both Statement I and Statement II are correct and Statement II is not the correct explanation of Statement I
- c)Statement I is correct but Statement II is incorrect
- d)Statement II is correct but Statement I is incorrect
Correct answer is option 'B'. Can you explain this answer?
Direction (Q. No. 9) This sectionis based on statement I and Statement II. Select the correct answer from the code given below.
Q.
Statement I :Every endothermic reaction is spontaneous if TΔS > ΔH.
Statement II : Sign of ΔG is the true criterion for deciding spontaneity of a reaction.
a)
Both Statement I and Statement II are correct and Statement II is the correct explanation of Statement I
b)
Both Statement I and Statement II are correct and Statement II is not the correct explanation of Statement I
c)
Statement I is correct but Statement II is incorrect
d)
Statement II is correct but Statement I is incorrect

|
Ambition Institute answered |
Statement I: Every endothermic reaction is spontaneous if TΔS > ΔH.
Endothermic reactions have a positive ΔH (heat absorbed). For spontaneity, we use the Gibbs free energy equation:
ΔG=ΔH−TΔS\Delta G = \Delta H - T\Delta SΔG=ΔH−TΔS For a process to be spontaneous, ΔG<0\Delta G < 0ΔG<0. If TΔS>ΔHT\Delta S > \Delta HTΔS>ΔH, then ΔG\Delta GΔG becomes negative, indicating spontaneity. Hence, Statement I is correct.
ΔG=ΔH−TΔS\Delta G = \Delta H - T\Delta SΔG=ΔH−TΔS For a process to be spontaneous, ΔG<0\Delta G < 0ΔG<0. If TΔS>ΔHT\Delta S > \Delta HTΔS>ΔH, then ΔG\Delta GΔG becomes negative, indicating spontaneity. Hence, Statement I is correct.
Statement II: Sign of ΔG is the true criterion for deciding spontaneity of a reaction.
This is a fundamental thermodynamic principle. The sign of ΔG\Delta GΔG indeed determines whether a reaction is spontaneous (ΔG<0\Delta G < 0ΔG<0) or non-spontaneous (ΔG>0\Delta G > 0ΔG>0). Hence, Statement II is also correct.
Correct Answer: Since both statements are correct and the second statement explains the first, the correct option is:
A: Both Statement I and Statement II are correct and Statement II is the correct explanation of Statement I.
Chapter doubts & questions for Energy Changes - Chemistry for JAMB 2025 is part of JAMB exam preparation. The chapters have been prepared according to the JAMB exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for JAMB 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Energy Changes - Chemistry for JAMB in English & Hindi are available as part of JAMB exam.
Download more important topics, notes, lectures and mock test series for JAMB Exam by signing up for free.
Chemistry for JAMB
213 videos|209 docs|162 tests
|

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup on EduRev and stay on top of your study goals
10M+ students crushing their study goals daily


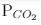 = 0.0030 atm.
= 0.0030 atm.









