All Exams >
EmSAT Achieve >
Chemistry for EmSAT Achieve >
All Questions
All questions of Lewis Structures for Atoms & Molecules for EmSAT Achieve Exam
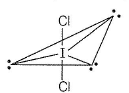
The shape of the below molecule is
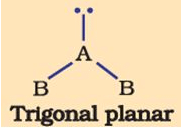
- a)trigonal
- b)rigonal planar
- c)see saw
- d)bent
Correct answer is option 'D'. Can you explain this answer?
The shape of the below molecule is

a)
trigonal
b)
rigonal planar
c)
see saw
d)
bent
|
|
Lavanya Menon answered |
The order of Repulsion: lone pair-lone pair > lone pair-bond pair > bond pair-bond pair
Due to the extra lone pair electron, the shape becomes bent.
Which of the following angle corresponds to sp2 hybridisation?a)120∘b)180∘c)90∘d)109∘Correct answer is option 'A'. Can you explain this answer?
|
|
Samridhi Pillai answered |
sp2 hybridisation gives three sp2 hybrid orbitals which are planar triangular forming an angle of 120° with each other.
The electronic configurations of three elements A, B and C are given below.
Answer the questions from 14 to 17 on the basis of these configurations.
A ls22s22p6
B ls22s22p63s23p3
C ls22s22p63s23p
The electronic configurations of three elements A, B and C are given below.
Answer the questions from 14 to 17 on the basis of these configurations.
A ls22s22p6
B ls22s22p63s23p3
C ls22s22p63s23p
bond lengths are lower in elements havinga)crystal structureb)double bondc)triple bondd)single bondCorrect answer is option 'C'. Can you explain this answer?
|
|
Rajesh Gupta answered |
Single bond has higher bond length than multiple bond.
Which is the correct order of the bond angle?- a)NH3 < NF3
- b)H2O > Cl2O
- c)PH3 < SbH3
- d)H2Te < H2S
Correct answer is option 'D'. Can you explain this answer?
Which is the correct order of the bond angle?
a)
NH3 < NF3
b)
H2O > Cl2O
c)
PH3 < SbH3
d)
H2Te < H2S

|
Nitin Patel answered |
The high electronegativity of F pulls the bonding electrons farther away from N than in NH3. Thus, repulsion between bond pairs is iess in NF3 than in NH3. Flence, the lone pair in N causes a greater distortion than NH3.
(b) as in (a)
Going down the group in periodic table, as size of central atom increases, repulsion increases.
Select the correct statement(s) about IF7.- a)I atom is sp3d3-hybridised
- b)I atom is in highest oxidation state
- c)There are five I—F longest and two I—F shortest bonds
- d)It has pentagonal bipyramidal structure
Correct answer is option 'A,B,C,D'. Can you explain this answer?
Select the correct statement(s) about IF7.
a)
I atom is sp3d3-hybridised
b)
I atom is in highest oxidation state
c)
There are five I—F longest and two I—F shortest bonds
d)
It has pentagonal bipyramidal structure
|
|
Rohit Shah answered |
Iodine heptafluoride, also known as iodine(VII) fluoride or iodine fluoride, is an interhalogen compound with the chemical formula IF7.[2][3] It has an unusual pentagonal bipyramidal structure, as predicted by VSEPR theory.[4] The molecule can undergo a pseudorotational rearrangement called the Bartell mechanism, which is like the Berry mechanism but for a heptacoordinated system.[5] It forms colourless crystals, which melt at 4.5 degC: the liquid range is extremely narrow, with the boiling point at 4.77 degC. The dense vapor has a mouldy, acrid odour. The molecule has D5h symmetry. In IF7 out of 7 Flourine atoms 5 of them are placed on a plane in Pentagon shape .In remaining 2 flourines one is placed above the plane and other below the plane each at 90 degrees
A qualitative measure of the stability of an ionic compound is provided- a)ionization enthalpy
- b)lattice enthalpy
- c)Electron affinity
- d)electron gain enthalpy
Correct answer is option 'B'. Can you explain this answer?
A qualitative measure of the stability of an ionic compound is provided
a)
ionization enthalpy
b)
lattice enthalpy
c)
Electron affinity
d)
electron gain enthalpy

|
Sounak Chaudhary answered |
stability of ionic bond is directly propotional to lattice energy.
Consider the two structures :
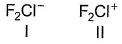
Select the correct statement(s).
a)I is bent, II is linearb)I is linear, II is bentc)Both is bentd)Both are linearCorrect answer is option 'B'. Can you explain this answer?
|
|
Geetika Shah answered |
1 has three lone pair and two bond pair, its representation will be AB2L3 and according to VSEPR theory it is linear in shape.
2 has two LP and two BP which representation will be AB2L2, so according to VSEPR theory it will be bent or it can also said to be 'V' shaped.
Hence B is correct.
Rank the following bonds in order of increasing polarity: H-N, H-O, H-C.- a)H-O < H-N < H-C
- b)H - C < H-N < H-O.
- c)H - C < H-O < H-N.
- d)H-N < H-O < H-C
Correct answer is option 'B'. Can you explain this answer?
Rank the following bonds in order of increasing polarity: H-N, H-O, H-C.
a)
H-O < H-N < H-C
b)
H - C < H-N < H-O.
c)
H - C < H-O < H-N.
d)
H-N < H-O < H-C

|
Rahul Chaudhary answered |
O is more electronegative than N which is more electronegative than C.
Hybridization of C2and C3of H3C −− CH = C = CH −− CH3are - a)Sp, Sp3
- b)Sp2, Sp2
- c)Sp2, Sp
- d)None of the above
Correct answer is option 'C'. Can you explain this answer?
Hybridization of C2and C3of H3C −− CH = C = CH −− CH3are
a)
Sp, Sp3
b)
Sp2, Sp2
c)
Sp2, Sp
d)
None of the above
|
|
Arun Khanna answered |
The first carbon atom forms four sigma bonds, three with Hydrogen and one with carbon. So, the carbon here is sp3 hybridised.The second carbon atom forms three sigma bonds and one pi bond. The three sigma bonds can be possible only when carbon is sp2 hybridised. The fourth electron forms a pi overlap with an electron from third carbon atom.
Carbon atom 3 forms two sigma bonds and is sp hybridised. The two p electrons form a pi bond with p electrons of the neighbouring carbon atoms.Carbon atom 4 is similar to carbon 2, forms 3 sigma bonds and is sp2 hybridised.
As the aldehyde formed has molar mass of 44u, so the aldehyde is acetaldehyde or ethanal. The alkene that gives rise to ethanal, is but-2-ene. Ozonolysis leads to breaking the alkene molecule into two molecules at the double bond. As only one product, ethanal is formed, two carbon atoms surround the two sides of the double bond.
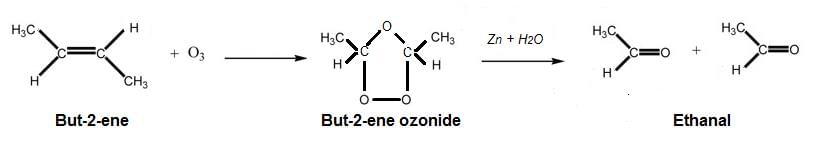
The number of types of bonds between two carbon atoms in calcium carbide is- a)Two sigma, two pi
- b)One sigma, two pi
- c)One sigma, one pi
- d)Two sigma, one pi
Correct answer is option 'B'. Can you explain this answer?
The number of types of bonds between two carbon atoms in calcium carbide is
a)
Two sigma, two pi
b)
One sigma, two pi
c)
One sigma, one pi
d)
Two sigma, one pi
|
|
Jyoti Kumar answered |
Explanation:
Calcium carbide is a chemical compound with the chemical formula CaC2. It is composed of calcium and two carbon atoms. The two carbon atoms in calcium carbide are bonded together, and the type of bond between them is determined by the way they share electrons.
The types of bonds between two carbon atoms in calcium carbide are:
1. Sigma Bond: A sigma bond is formed when two atoms overlap their atomic orbitals end to end, creating a single bond. In calcium carbide, there is one sigma bond between the two carbon atoms.
2. Pi Bond: A pi bond is formed when two atoms share electrons in parallel orbitals that overlap above and below the internuclear axis. In calcium carbide, there are two pi bonds between the two carbon atoms.
Therefore, the correct answer is option B, which is one sigma bond and two pi bonds between the two carbon atoms in calcium carbide.
Calcium carbide is a chemical compound with the chemical formula CaC2. It is composed of calcium and two carbon atoms. The two carbon atoms in calcium carbide are bonded together, and the type of bond between them is determined by the way they share electrons.
The types of bonds between two carbon atoms in calcium carbide are:
1. Sigma Bond: A sigma bond is formed when two atoms overlap their atomic orbitals end to end, creating a single bond. In calcium carbide, there is one sigma bond between the two carbon atoms.
2. Pi Bond: A pi bond is formed when two atoms share electrons in parallel orbitals that overlap above and below the internuclear axis. In calcium carbide, there are two pi bonds between the two carbon atoms.
Therefore, the correct answer is option B, which is one sigma bond and two pi bonds between the two carbon atoms in calcium carbide.
XeF2 is isostructural with- a)TeF2
- b)
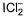
- c)SbCI3
- d)BaCl2
Correct answer is option 'B'. Can you explain this answer?
XeF2 is isostructural with
a)
TeF2
b)
c)
SbCI3
d)
BaCl2
|
|
Varun Kapoor answered |
XeF2-sp3d hybridised Xe
Three lone pairs on equatorial positions to minimise repulsion; two F-atom at axial position. Thus, it is linear.
(a) TeF2,Te50 - Six electrons in valence shell
(b)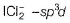 hybridised l- atom with three lone pairs at equatorial positions to minimise repulsion thus, linear.
hybridised l- atom with three lone pairs at equatorial positions to minimise repulsion thus, linear.
(c) SoCI3 - sp3-hybridised pyramidal Sb-one lone pair on Sb.
(d) BaCI2 - ionic .
Three lone pairs on equatorial positions to minimise repulsion; two F-atom at axial position. Thus, it is linear.
(a) TeF2,Te50 - Six electrons in valence shell
(b)
(c) SoCI3 - sp3-hybridised pyramidal Sb-one lone pair on Sb.
(d) BaCI2 - ionic .
When a gas phase atom in its ground state gains an electron. This is called- a)electron gain enthalpy
- b)ionization enthalpy
- c)Electron affinity
- d)lattice enthalpy
Correct answer is option 'A'. Can you explain this answer?
When a gas phase atom in its ground state gains an electron. This is called
a)
electron gain enthalpy
b)
ionization enthalpy
c)
Electron affinity
d)
lattice enthalpy
|
|
Anirban Shah answered |
Explanation:
When a gas phase atom in its ground state gains an electron, it undergoes a process known as electron gain enthalpy.
Electron Gain Enthalpy:
Electron gain enthalpy is defined as the energy change that occurs when a neutral gaseous atom gains an electron to form a negative ion. It is a measure of the ability of an atom to attract and accept an electron.
Factors affecting Electron Gain Enthalpy:
The electron gain enthalpy of an atom depends on several factors:
1. Atomic size: Smaller atoms have a higher electron gain enthalpy as the incoming electron experiences a stronger attractive force from the nucleus.
2. Nuclear charge: Higher nuclear charge results in a higher electron gain enthalpy as the attractive force between the nucleus and the incoming electron is stronger.
3. Electronic configuration: If the incoming electron enters a completely filled or a stable configuration, it requires more energy to add an electron, resulting in a higher electron gain enthalpy.
4. Shielding effect: Greater shielding effect by inner electrons reduces the attraction between the nucleus and the incoming electron, leading to a lower electron gain enthalpy.
Significance:
The electron gain enthalpy is an important concept in chemistry as it helps in understanding the reactivity and chemical behavior of elements. Elements with higher electron gain enthalpy tend to have a greater affinity for electrons and are more likely to form negative ions. On the other hand, elements with lower electron gain enthalpy have a lower tendency to gain electrons and are more likely to form positive ions.
Answer:
Therefore, when a gas phase atom in its ground state gains an electron, the process is referred to as electron gain enthalpy.
When a gas phase atom in its ground state gains an electron, it undergoes a process known as electron gain enthalpy.
Electron Gain Enthalpy:
Electron gain enthalpy is defined as the energy change that occurs when a neutral gaseous atom gains an electron to form a negative ion. It is a measure of the ability of an atom to attract and accept an electron.
Factors affecting Electron Gain Enthalpy:
The electron gain enthalpy of an atom depends on several factors:
1. Atomic size: Smaller atoms have a higher electron gain enthalpy as the incoming electron experiences a stronger attractive force from the nucleus.
2. Nuclear charge: Higher nuclear charge results in a higher electron gain enthalpy as the attractive force between the nucleus and the incoming electron is stronger.
3. Electronic configuration: If the incoming electron enters a completely filled or a stable configuration, it requires more energy to add an electron, resulting in a higher electron gain enthalpy.
4. Shielding effect: Greater shielding effect by inner electrons reduces the attraction between the nucleus and the incoming electron, leading to a lower electron gain enthalpy.
Significance:
The electron gain enthalpy is an important concept in chemistry as it helps in understanding the reactivity and chemical behavior of elements. Elements with higher electron gain enthalpy tend to have a greater affinity for electrons and are more likely to form negative ions. On the other hand, elements with lower electron gain enthalpy have a lower tendency to gain electrons and are more likely to form positive ions.
Answer:
Therefore, when a gas phase atom in its ground state gains an electron, the process is referred to as electron gain enthalpy.
H.O.H bond angle in water is- a)1100
- b)2400
- c)1040
- d)4160
Correct answer is option 'C'. Can you explain this answer?
H.O.H bond angle in water is
a)
1100
b)
2400
c)
1040
d)
4160

|
Aravind Saha answered |
Due to presence of two lone pairs on O in H2O bond angle reduce to 104 from 109.
The formation of the Cl2 molecule can be understood in terms of the sharing of a pair of electrons between the two chlorine atoms, each chlorine atom contributing one electron to the shared pair. Choose the most appropriate name of the bond that is formed:
- a)Double bond
- b)Multiple bond
- c)Single covalent bond
- d)Ionic bond
Correct answer is option 'C'. Can you explain this answer?
The formation of the Cl2 molecule can be understood in terms of the sharing of a pair of electrons between the two chlorine atoms, each chlorine atom contributing one electron to the shared pair. Choose the most appropriate name of the bond that is formed:
a)
Double bond
b)
Multiple bond
c)
Single covalent bond
d)
Ionic bond

|
Rishabh Das answered |
Cl2 is formed by single covalent bond.
Rank the bonds in the set C=O, C-O, C≡O in order of decreasing bond length- a)C-O > C≡O > C=O
- b)C-O > C=O > C≡O
- c)C≡O< C-O > C=O
- d)none
Correct answer is option 'B'. Can you explain this answer?
Rank the bonds in the set C=O, C-O, C≡O in order of decreasing bond length
a)
C-O > C≡O > C=O
b)
C-O > C=O > C≡O
c)
C≡O< C-O > C=O
d)
none
|
|
Preeti Khanna answered |
C≡O has triple bond so has minimum bond length.
Elements in which apart from 3s and 3p orbitals, 3d orbitals also available for bonding In a number of compounds of these elements there are more than eight valence electrons around the central atom. One such example is
- a)H2O
- b)HCl
- c)HNO3
- d)H2SO4
Correct answer is option 'D'. Can you explain this answer?
Elements in which apart from 3s and 3p orbitals, 3d orbitals also available for bonding In a number of compounds of these elements there are more than eight valence electrons around the central atom. One such example is
a)
H2O
b)
HCl
c)
HNO3
d)
H2SO4
|
|
Suresh Iyer answered |
In H2SO4, S has more than 8 electrons in the valence shell.
Direction (Q. Nos. 1-16) This section contains 16 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.Q. The shape [CIF4]- and [ClF2]- ions is respectively- a)see-saw and linear
- b)see-saw and bent
- c)tetrahedral and linear
- d)square planar and linear
Correct answer is option 'D'. Can you explain this answer?
Direction (Q. Nos. 1-16) This section contains 16 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Q. The shape [CIF4]- and [ClF2]- ions is respectively
a)
see-saw and linear
b)
see-saw and bent
c)
tetrahedral and linear
d)
square planar and linear
|
|
Raghav Bansal answered |
Direction (Q. Nos. 25 and 26) This section contains 2 questions. when worked out will result in an integer from 0 to 9 (both inclusive).Q. i. Based on VSEPR theory, the number of 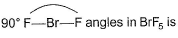
ii. Based on VSEPR theory, number of lone pairs in XeF2 is......
iii. The total number of lone pair of electrons in melamine i s ......[JEE Advanced 2013]
Correct answer is '0'. Can you explain this answer?
Direction (Q. Nos. 25 and 26) This section contains 2 questions. when worked out will result in an integer from 0 to 9 (both inclusive).
Q.
i. Based on VSEPR theory, the number of 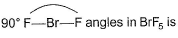
ii. Based on VSEPR theory, number of lone pairs in XeF2 is......
iii. The total number of lone pair of electrons in melamine i s ......
ii. Based on VSEPR theory, number of lone pairs in XeF2 is......
iii. The total number of lone pair of electrons in melamine i s ......
[JEE Advanced 2013]
|
|
Geetika Shah answered |
(i) (0)
(Ip-bp) repulsion will cause distortion and thus, bond angle < 90° in each.
Thus, three lone pairs at equatorial position to minimise repulsion.
(iii) (6) Melamine is heterocyclic basic com pound (six N-atoms) each N having one lone pair.
Among the following the maximum covalent character is shown by the compound- a)MgCl2
- b)FeCl2
- c)SnCl2
- d)AlCl3
Correct answer is option 'D'. Can you explain this answer?
Among the following the maximum covalent character is shown by the compound
a)
MgCl2
b)
FeCl2
c)
SnCl2
d)
AlCl3
|
|
Anjali Iyer answered |
The proportion of covalent character in an ionic bond is decided by polarisability of the metal cation as well as the electronegativity of both elements involved in bonding. Polarisability is further decided by the density of positive charge on the metal cation. AICI3 is considered to show maximum covalent character among the given compounds. This is because Al 3 + bears 3 unit of positive charge and shows strong tendency to distort the electron cloud, thus the covalent character in Al-CI bond dramatically increases.
Which of the following statements are not correct?
- a)In canonical structures there is a difference in the arrangement of atoms.
- b)Hybrid orbitals form stronger bonds than pure orbitals.
- c)NaCl being an ionic compound is a good conductor of electricity in the solid state.
- d)VSEPR Theory can explain the square planar geometry of XeF4.
Correct answer is option 'A,C'. Can you explain this answer?
Which of the following statements are not correct?
a)
In canonical structures there is a difference in the arrangement of atoms.
b)
Hybrid orbitals form stronger bonds than pure orbitals.
c)
NaCl being an ionic compound is a good conductor of electricity in the solid state.
d)
VSEPR Theory can explain the square planar geometry of XeF4.

|
Athul Yadav answered |
In NaCl the ions are not free in solid state but they are strongly bonded through electrostatic forces, hence is not a good conductor of electricity in solid state
A canonicalstructure is also known as a resonance structure, i.e. one of possibly more than one contributing structures that combine to produce the true, resonance hybrid structure. The arrangement of atoms remains the same.
Correct option is A and C.
A canonicalstructure is also known as a resonance structure, i.e. one of possibly more than one contributing structures that combine to produce the true, resonance hybrid structure. The arrangement of atoms remains the same.
Correct option is A and C.
Which one of the following is paramagnetic?- a)NO
- b)O3
- c)CO
- d)N2
Correct answer is option 'A'. Can you explain this answer?
Which one of the following is paramagnetic?
a)
NO
b)
O3
c)
CO
d)
N2

|
Raghav Shah answered |
NO is a odd electron species.
In BF3BF3, molecule below the dipole moment is zero although the B- F bonds are oriented at an angle of 1200 to one another. Net dipole moment in BF3, molecule is- a)three
- b)two
- c)zero
- d)one
Correct answer is option 'C'. Can you explain this answer?
In BF3BF3, molecule below the dipole moment is zero although the B- F bonds are oriented at an angle of 1200 to one another. Net dipole moment in BF3, molecule is
a)
three
b)
two
c)
zero
d)
one

|
Naveen Choudhary answered |
In BF3, molecule above the dipole moment is zero because the B- F bonds are oriented at an angle of 1200 to one another so net dipole cancel out.
The pi-bond involves __________- a)axial overlapping
- b)side-wise overlapping
- c)end to end type of overlapping
- d)head-on overlapping
Correct answer is option 'B'. Can you explain this answer?
The pi-bond involves __________
a)
axial overlapping
b)
side-wise overlapping
c)
end to end type of overlapping
d)
head-on overlapping
|
|
Hansa Sharma answered |
A pi-bond is a type of covalent bond in which the internuclear axes of the atoms are parallel to each other and for side-wise overlapping. The bond formed here is perpendicular to the internuclear axes.
What is the electronic configuration of carbon in it’s excited state?- a)1s22s22p4
- b)1s22s12p3
- c)1s22s22p5
- d)1s22s12p4
Correct answer is option 'B'. Can you explain this answer?
What is the electronic configuration of carbon in it’s excited state?
a)
1s22s22p4
b)
1s22s
1
2p3c)
1s22s22p5
d)
1s22s12p4
|
|
Amar Mehra answered |
Understanding Carbon's Electron Configuration
Carbon, with an atomic number of 6, has a ground state electronic configuration of 1s² 2s² 2p². In this configuration, all electrons are in their lowest energy states. However, during an excited state, one or more electrons can absorb energy and jump to higher energy levels.
Excited State Configuration
- In the excited state, one of the 2s electrons can absorb energy and move to the 2p orbital.
- This results in the following configuration: 1s² 2s¹ 2p³.
Options Analysis
Let's evaluate the given options:
- a) 1s² 2s² 2p⁴: This suggests an extra electron in the 2p level, which does not correspond to carbon's excited state.
- b) 1s² 2s¹ 2p³: This arrangement accurately reflects the excitation process where one electron from 2s moves to 2p.
- c) 1s² 2s² 2p⁵: This configuration implies an additional electron in 2p, which is incorrect for carbon.
- d) 1s² 2s¹ 2p⁴: This indicates two electrons in 2p, which is not possible for carbon in its excited state.
Conclusion
The correct answer is option b) 1s² 2s¹ 2p³, as it accurately represents the excited state of carbon where one electron from the 2s orbital has moved to the 2p orbital. This configuration allows carbon to participate in different chemical bonding scenarios, showcasing its versatility in forming compounds.
Carbon, with an atomic number of 6, has a ground state electronic configuration of 1s² 2s² 2p². In this configuration, all electrons are in their lowest energy states. However, during an excited state, one or more electrons can absorb energy and jump to higher energy levels.
Excited State Configuration
- In the excited state, one of the 2s electrons can absorb energy and move to the 2p orbital.
- This results in the following configuration: 1s² 2s¹ 2p³.
Options Analysis
Let's evaluate the given options:
- a) 1s² 2s² 2p⁴: This suggests an extra electron in the 2p level, which does not correspond to carbon's excited state.
- b) 1s² 2s¹ 2p³: This arrangement accurately reflects the excitation process where one electron from 2s moves to 2p.
- c) 1s² 2s² 2p⁵: This configuration implies an additional electron in 2p, which is incorrect for carbon.
- d) 1s² 2s¹ 2p⁴: This indicates two electrons in 2p, which is not possible for carbon in its excited state.
Conclusion
The correct answer is option b) 1s² 2s¹ 2p³, as it accurately represents the excited state of carbon where one electron from the 2s orbital has moved to the 2p orbital. This configuration allows carbon to participate in different chemical bonding scenarios, showcasing its versatility in forming compounds.
Following reaction, CIF3 + AsF5 → (CIF2+ ) (AsF6-)Q. Select the correct statement(s).- a)CIF3 changes its structure from pyramidal to angular in CIF+2
- b)AsF5 changes to structure from angular bipyramidal to octahedral in (AsF6)-
- c)Both (a) and (b) are correct
- d)None of the above is correct
Correct answer is option 'B'. Can you explain this answer?
Following reaction, CIF3 + AsF5 → (CIF2+ ) (AsF6-)
Q.
Select the correct statement(s).
a)
CIF3 changes its structure from pyramidal to angular in CIF+2
b)
AsF5 changes to structure from angular bipyramidal to octahedral in (AsF6)-
c)
Both (a) and (b) are correct
d)
None of the above is correct
|
|
Muskaan Kumar answered |
Which of the following sets of molecules have different shape but same hybridisation of the central atoms?
- a)NH3, H2O
- b)H2S, SO2
- c)Ni(CO)4,[NiCI4]2-
- d)SF4, PCI5
Correct answer is option 'A,D'. Can you explain this answer?
Which of the following sets of molecules have different shape but same hybridisation of the central atoms?
a)
NH3, H2O
b)
H2S, SO2
c)
Ni(CO)4,[NiCI4]2-
d)
SF4, PCI5

|
Devika Banerjee answered |
Explanation:
In order to determine whether molecules have the same hybridization of the central atoms, we need to consider the geometry and the number of electron regions around the central atom. The hybridization of an atom is determined by the number of electron regions (bonding pairs and lone pairs) around it.
Option A: NH3, H2O
- NH3: The central atom is nitrogen. It has three bonding pairs and one lone pair. The electron regions are arranged in a trigonal pyramidal geometry. The hybridization of nitrogen in NH3 is sp3.
- H2O: The central atom is oxygen. It has two bonding pairs and two lone pairs. The electron regions are arranged in a bent or V-shaped geometry. The hybridization of oxygen in H2O is also sp3.
Option B: H2S, SO2
- H2S: The central atom is sulfur. It has two bonding pairs and two lone pairs. The electron regions are arranged in a bent or V-shaped geometry. The hybridization of sulfur in H2S is sp3.
- SO2: The central atom is sulfur. It has two bonding pairs and one lone pair. The electron regions are arranged in a bent or V-shaped geometry. The hybridization of sulfur in SO2 is also sp3.
Option C: Ni(CO)4, [NiCl4]2-
- Ni(CO)4: The central atom is nickel. It has four bonding pairs. The electron regions are arranged in a tetrahedral geometry. The hybridization of nickel in Ni(CO)4 is sp3.
- [NiCl4]2-: The central atom is nickel. It has four bonding pairs. The electron regions are arranged in a tetrahedral geometry. The hybridization of nickel in [NiCl4]2- is also sp3.
Option D: SF4, PCl5
- SF4: The central atom is sulfur. It has four bonding pairs and one lone pair. The electron regions are arranged in a trigonal bipyramidal geometry. The hybridization of sulfur in SF4 is sp3d.
- PCl5: The central atom is phosphorus. It has five bonding pairs. The electron regions are arranged in a trigonal bipyramidal geometry. The hybridization of phosphorus in PCl5 is sp3d.
Conclusion:
From the analysis, it can be concluded that options A (NH3, H2O) and D (SF4, PCl5) have different shapes but the same hybridization of the central atoms.
In order to determine whether molecules have the same hybridization of the central atoms, we need to consider the geometry and the number of electron regions around the central atom. The hybridization of an atom is determined by the number of electron regions (bonding pairs and lone pairs) around it.
Option A: NH3, H2O
- NH3: The central atom is nitrogen. It has three bonding pairs and one lone pair. The electron regions are arranged in a trigonal pyramidal geometry. The hybridization of nitrogen in NH3 is sp3.
- H2O: The central atom is oxygen. It has two bonding pairs and two lone pairs. The electron regions are arranged in a bent or V-shaped geometry. The hybridization of oxygen in H2O is also sp3.
Option B: H2S, SO2
- H2S: The central atom is sulfur. It has two bonding pairs and two lone pairs. The electron regions are arranged in a bent or V-shaped geometry. The hybridization of sulfur in H2S is sp3.
- SO2: The central atom is sulfur. It has two bonding pairs and one lone pair. The electron regions are arranged in a bent or V-shaped geometry. The hybridization of sulfur in SO2 is also sp3.
Option C: Ni(CO)4, [NiCl4]2-
- Ni(CO)4: The central atom is nickel. It has four bonding pairs. The electron regions are arranged in a tetrahedral geometry. The hybridization of nickel in Ni(CO)4 is sp3.
- [NiCl4]2-: The central atom is nickel. It has four bonding pairs. The electron regions are arranged in a tetrahedral geometry. The hybridization of nickel in [NiCl4]2- is also sp3.
Option D: SF4, PCl5
- SF4: The central atom is sulfur. It has four bonding pairs and one lone pair. The electron regions are arranged in a trigonal bipyramidal geometry. The hybridization of sulfur in SF4 is sp3d.
- PCl5: The central atom is phosphorus. It has five bonding pairs. The electron regions are arranged in a trigonal bipyramidal geometry. The hybridization of phosphorus in PCl5 is sp3d.
Conclusion:
From the analysis, it can be concluded that options A (NH3, H2O) and D (SF4, PCl5) have different shapes but the same hybridization of the central atoms.
Quartz is very hard and melts at 1550∘C . Reason is:- a)separate Si02 molecules exist they are held together by coordinate bonds that extend throughout the sample
- b)separate Si02 molecules exist that are held together by pi bonds that extend in three dimensions throughout the sample
- c)Quartz (Si02) has silicon-oxygen electrovalent bonds that extend throughout the sample
- d)Quartz (Si02) has silicon-oxygen covalent bonds that extend throughout the sample
Correct answer is option 'D'. Can you explain this answer?
Quartz is very hard and melts at 1550∘C . Reason is:
a)
separate Si02 molecules exist they are held together by coordinate bonds that extend throughout the sample
b)
separate Si02 molecules exist that are held together by pi bonds that extend in three dimensions throughout the sample
c)
Quartz (Si02) has silicon-oxygen electrovalent bonds that extend throughout the sample
d)
Quartz (Si02) has silicon-oxygen covalent bonds that extend throughout the sample

|
Sparsh Sen answered |
Quartz/silica have extended covalent bond network.
CO is isoelectronic with- a)SnCl2and NO+
- b)NO−2 and SnCl2
- c)NO+and N2
- d)N2and SnCl2
Correct answer is option 'C'. Can you explain this answer?
CO is isoelectronic with
a)
SnCl2and NO+
b)
NO−2 and SnCl2
c)
NO+and N2
d)
N2and SnCl2

|
Prisha Yadav answered |
CO is isoelectronic with a) SnCl2 and NO.
Isoelectronic species are those that have the same number of electrons and therefore have similar electronic configurations.
CO has a total of 14 electrons.
a) SnCl2: Sn has an electron configuration of [Kr]5s24d105p2. It loses 2 electrons to form Sn2+, giving it a configuration of [Kr]5s24d10. Cl has an electron configuration of [Ne]3s23p5. Each Cl atom gains 1 electron to form Cl-, giving it a configuration of [Ne]3s23p6. Therefore, SnCl2 has a total of 14 electrons like CO, making them isoelectronic.
b) NO: N has an electron configuration of [He]2s22p3. It gains 2 electrons to form N2-, giving it a configuration of [He]2s22p6. O has an electron configuration of [He]2s22p4. It gains 2 electrons to form O2-, giving it a configuration of [He]2s22p6. Therefore, NO has a total of 14 electrons like CO, making them isoelectronic.
Isoelectronic species are those that have the same number of electrons and therefore have similar electronic configurations.
CO has a total of 14 electrons.
a) SnCl2: Sn has an electron configuration of [Kr]5s24d105p2. It loses 2 electrons to form Sn2+, giving it a configuration of [Kr]5s24d10. Cl has an electron configuration of [Ne]3s23p5. Each Cl atom gains 1 electron to form Cl-, giving it a configuration of [Ne]3s23p6. Therefore, SnCl2 has a total of 14 electrons like CO, making them isoelectronic.
b) NO: N has an electron configuration of [He]2s22p3. It gains 2 electrons to form N2-, giving it a configuration of [He]2s22p6. O has an electron configuration of [He]2s22p4. It gains 2 electrons to form O2-, giving it a configuration of [He]2s22p6. Therefore, NO has a total of 14 electrons like CO, making them isoelectronic.
The bond enthalpy of ___________ molecule is 435.8 kJ mol-1.- a)Hydrogen
- b)Oxygen
- c)Nitrogen
- d)Helium
Correct answer is option 'A'. Can you explain this answer?
The bond enthalpy of ___________ molecule is 435.8 kJ mol-1.
a)
Hydrogen
b)
Oxygen
c)
Nitrogen
d)
Helium
|
|
Hansa Sharma answered |
The amount of energy that is required to break a chemical bond in a molecule into individual atoms is known as bond enthalpy. 435.8 kJ mol-1 is required to dissociate a hydrogen molecule into two hydrogen atoms.
Which of the following is not a homonuclear diatomic molecule?- a)H2
- b)N2
- c)O2
- d)HCl
Correct answer is option 'D'. Can you explain this answer?
Which of the following is not a homonuclear diatomic molecule?
a)
H2
b)
N2
c)
O2
d)
HCl
|
|
Neha Sharma answered |
The molecule that is formed from the same element is known as a homonuclear molecule and the molecule that is made up of 2 atoms is called a diatomic molecule. But HCl is not a homonuclear diatomic molecule as it has different atoms.
Which type of bond is present between hydrogens in hydrogen molecule?- a)Sigma bond
- b)Pi bond
- c)Ionic bond
- d)Metallic bond
Correct answer is option 'A'. Can you explain this answer?
Which type of bond is present between hydrogens in hydrogen molecule?
a)
Sigma bond
b)
Pi bond
c)
Ionic bond
d)
Metallic bond
|
|
Neha Sharma answered |
The head-on or end to end type of overlapping is present in sigma bond. A sigma bond is a type of covalent bond. It may also be called an axial overlap. In case of the hydrogen molecule, its s-s overlapping.
Direction (Q. Nos. 21-22) This section contains a paragraph, wach describing theory, experiments, data etc. three Questions related to paragraph have been given.Each question have only one correct answer among the four given options (a),(b),(c),(d).
Following reaction, CIF3 + AsF5 → (CIF2+ ) (AsF-6)Q. Select the correct statements.- a)Hybridisation of Cl changes from sp2 to sp
- b)Hybridisation of As changes from sp3d to sp3d2
- c)Both (a) and (b) are correct
- d)None of the above is correct
Correct answer is option 'B'. Can you explain this answer?
Direction (Q. Nos. 21-22) This section contains a paragraph, wach describing theory, experiments, data etc. three Questions related to paragraph have been given.Each question have only one correct answer among the four given options (a),(b),(c),(d).
Following reaction, CIF3 + AsF5 → (CIF2+ ) (AsF-6)
Q.
Select the correct statements.
a)
Hybridisation of Cl changes from sp2 to sp
b)
Hybridisation of As changes from sp3d to sp3d2
c)
Both (a) and (b) are correct
d)
None of the above is correct
|
|
Om Desai answered |
Two of the following species have same shape 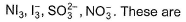
- a)
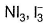
- b)
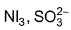
- c)
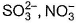
- d)
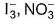
Correct answer is option 'B'. Can you explain this answer?
Two of the following species have same shape
a)
b)
c)
d)
|
|
Pooja Shah answered |
Both have one lone pair-trigonal pyramidal.
Using MO theory predicts which of the following species has the shortest bond length? - a)O+2
- b)O−2
- c)O2+2
- d)O2−2
Correct answer is option 'C'. Can you explain this answer?
Using MO theory predicts which of the following species has the shortest bond length?
a)
O+2
b)
O−2
c)
O2+2
d)
O2−2
|
|
Rahul Bansal answered |
O^2+2 has highest bond order 3 compare to other molecules, hence it has the shortest bond length.
Rank the bonds in the set C=O, C-O, C≡O in order of decreasing bond strength
- a)C≡O > C-O < C=O
- b)C≡O > C=O > C-O
- c)C-O < C≡O > C=O
- d)C=O < C≡O > C-O
Correct answer is option 'B'. Can you explain this answer?
Rank the bonds in the set C=O, C-O, C≡O in order of decreasing bond strength
a)
C≡O > C-O < C=O
b)
C≡O > C=O > C-O
c)
C-O < C≡O > C=O
d)
C=O < C≡O > C-O

|
Devansh Goyal answered |
bond order increases bond strength also increases.
A __________ overlap doesn’t result in the formation of a bond.- a)positive
- b)negative
- c)zero
- d)rational
Correct answer is option 'C'. Can you explain this answer?
A __________ overlap doesn’t result in the formation of a bond.
a)
positive
b)
negative
c)
zero
d)
rational
|
|
Raghav Bansal answered |
Zero overlap means that the orbitals don’t overlap at all. When there is no overlapping the bond formation doesn’t occur. As we all know that the extent of overlapping is dependent on the strength of the bond.
s-orbitals are nondirectional because of- a)spherical symmetry
- b)their small size
- c)being first orbital
- d)All of the above
Correct answer is option 'A'. Can you explain this answer?
s-orbitals are nondirectional because of
a)
spherical symmetry
b)
their small size
c)
being first orbital
d)
All of the above
|
|
Neha Chakraborty answered |
Explanation:
S-orbitals are the spherical-shaped orbitals and are designated as 1s, 2s, 3s, and so on. They are present in the first energy level and other higher energy levels.
The s-orbitals are nondirectional because of the following reasons:
Spherical Symmetry:
- The s-orbitals are spherical in shape and have the same probability of finding an electron in any direction from the nucleus.
- Due to this, the electrons in s-orbitals are equally distributed in all the directions around the nucleus.
- Thus, s-orbitals are considered to be nondirectional in nature.
Small Size:
- The s-orbitals are smaller in size as compared to the p, d, and f orbitals.
- Due to their small size, they do not have any specific direction of orientation.
Being First Orbital:
- The s-orbitals are the first orbitals to be filled in an atom.
- As they are the first to be filled, they do not have any other orbitals to interact with, which makes them nondirectional.
Conclusion:
Hence, we can conclude that the s-orbitals are nondirectional due to their spherical symmetry, small size, and being the first orbital to be filled in an atom.
S-orbitals are the spherical-shaped orbitals and are designated as 1s, 2s, 3s, and so on. They are present in the first energy level and other higher energy levels.
The s-orbitals are nondirectional because of the following reasons:
Spherical Symmetry:
- The s-orbitals are spherical in shape and have the same probability of finding an electron in any direction from the nucleus.
- Due to this, the electrons in s-orbitals are equally distributed in all the directions around the nucleus.
- Thus, s-orbitals are considered to be nondirectional in nature.
Small Size:
- The s-orbitals are smaller in size as compared to the p, d, and f orbitals.
- Due to their small size, they do not have any specific direction of orientation.
Being First Orbital:
- The s-orbitals are the first orbitals to be filled in an atom.
- As they are the first to be filled, they do not have any other orbitals to interact with, which makes them nondirectional.
Conclusion:
Hence, we can conclude that the s-orbitals are nondirectional due to their spherical symmetry, small size, and being the first orbital to be filled in an atom.
Among the following, the species having the smallest bond length is:
- a)NO+
- b)NO
- c)O2
- d)NO–
Correct answer is option 'A'. Can you explain this answer?
Among the following, the species having the smallest bond length is:
a)
NO+
b)
NO
c)
O2
d)
NO–

|
Sahil Saha answered |
The bond order of given molecules are:
NO = 2.5, NO+ = 3, O2 = 2, NO– = 2
Larger the bond order, the smaller the bond length.
NO+ has the largest bond order 3.
Therefore, it will have the smallest bond length.
Hence option A is the answer.
NO = 2.5, NO+ = 3, O2 = 2, NO– = 2
Larger the bond order, the smaller the bond length.
NO+ has the largest bond order 3.
Therefore, it will have the smallest bond length.
Hence option A is the answer.
The strength of covalent ___________ extent of overlapping of orbitals.- a)may be or may not be related
- b)is independent on
- c)is dependent on
- d)is not related to
Correct answer is option 'C'. Can you explain this answer?
The strength of covalent ___________ extent of overlapping of orbitals.
a)
may be or may not be related
b)
is independent on
c)
is dependent on
d)
is not related to
|
|
Geetika Shah answered |
As per the concept of valence bond theory, the partial merging of atomic orbitals id knowns as overlapping. The extent of overlapping is directly proportional to the strength of the covalent bond, i.e. it is dependent.
A positive overlap is same as ________- a)out-phase overlap
- b)negative overlap
- c)zero overlap
- d)in-phase overlap
Correct answer is option 'D'. Can you explain this answer?
A positive overlap is same as ________
a)
out-phase overlap
b)
negative overlap
c)
zero overlap
d)
in-phase overlap
|
|
Neha Sharma answered |
A positive overlap results in bond formation. When 2 p-orbitals are in phase, both the positive lobes overlap, thus creating a positive overlap and result in the bond formation, thus it is called in-phase overlap.
For which of the following sets of geometry, both axial and equatorial positions are present?- a)Octahedral, trigonal bipyramidal
- b)Tetrahedral, pentagonal bipyramidal
- c)Trigonal bipyramidal, pentagonal bipyramidal
- d)Tetrahedral, octahedral
Correct answer is option 'C'. Can you explain this answer?
For which of the following sets of geometry, both axial and equatorial positions are present?
a)
Octahedral, trigonal bipyramidal
b)
Tetrahedral, pentagonal bipyramidal
c)
Trigonal bipyramidal, pentagonal bipyramidal
d)
Tetrahedral, octahedral

|
Pallavi Banerjee answered |
Molecular shape of CF4, SF4 and XeF4 are- a)the same, with 2, 0 and 1 lone pair of electrons respectively
- b)the same with 1, 1 and 1 lone pair of electrons
- c)different, with 0, 1 and 2 lone pair of electrons
- d)different, with 1, 0 and 2 lone pair of electrons
Correct answer is option 'C'. Can you explain this answer?
Molecular shape of CF4, SF4 and XeF4 are
a)
the same, with 2, 0 and 1 lone pair of electrons respectively
b)
the same with 1, 1 and 1 lone pair of electrons
c)
different, with 0, 1 and 2 lone pair of electrons
d)
different, with 1, 0 and 2 lone pair of electrons
|
|
Mira Sharma answered |
SF4 molecule has 1 lone pair of electrons, CF4 has no lone pair of electrons and XeF4 has 2 lone pair of electrons respectively.
ECI3 (where, E = B, P, As, Bi) of these elements are known.
Bond angles 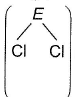 are in the following order
are in the following order- a)B > P> As > Bi
- b)B > P = As > Bi
- c)Bi > As > P > B
- d)B > As > Bi > P
Correct answer is option 'A'. Can you explain this answer?
ECI3 (where, E = B, P, As, Bi) of these elements are known.
Bond angles are in the following order
are in the following order
Bond angles
a)
B > P> As > Bi
b)
B > P = As > Bi
c)
Bi > As > P > B
d)
B > As > Bi > P

|
Akanksha Yadav answered |
Has no lone pair thus, bond angle is 120°. B-atom is sp2-hybridised.
Central atom E is sp3-hybridised. Hence, angle < 120°. As we go down the group, (Ip-bp) repulsion decreases. Hence, angle
(Cl—E—Cl) PCI3 > AsCI3 > BiCI3.
Thus, order is BCI3 > PCI3 > AsCI3 > BiCI3.
Chapter doubts & questions for Lewis Structures for Atoms & Molecules - Chemistry for EmSAT Achieve 2025 is part of EmSAT Achieve exam preparation. The chapters have been prepared according to the EmSAT Achieve exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for EmSAT Achieve 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Lewis Structures for Atoms & Molecules - Chemistry for EmSAT Achieve in English & Hindi are available as part of EmSAT Achieve exam.
Download more important topics, notes, lectures and mock test series for EmSAT Achieve Exam by signing up for free.
Chemistry for EmSAT Achieve
191 videos|265 docs|160 tests
|

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up within 7 days!
Access 1000+ FREE Docs, Videos and Tests
Takes less than 10 seconds to signup