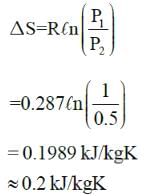All Exams >
UPSC >
Mechanical Engineering Optional Notes for UPSC >
All Questions
All questions of Basic Concepts & Zeroth Law of Thermodynamics for UPSC CSE Exam
Air undergoes a polytropic process in an adiabatic nozzle with n = 1.2. Inlet state of air is 800 kPa, 1230C with a velocity of 32 m/s and exit state is 300 kPa. Find out the velocity of air at nozzle exit.
- a)654 m/s
- b)348 m/s
- c)244 m/s
- d)436 m/s
Correct answer is option 'B'. Can you explain this answer?
Air undergoes a polytropic process in an adiabatic nozzle with n = 1.2. Inlet state of air is 800 kPa, 1230C with a velocity of 32 m/s and exit state is 300 kPa. Find out the velocity of air at nozzle exit.
a)
654 m/s
b)
348 m/s
c)
244 m/s
d)
436 m/s

|
Achuth Kumar Yelleti answered |
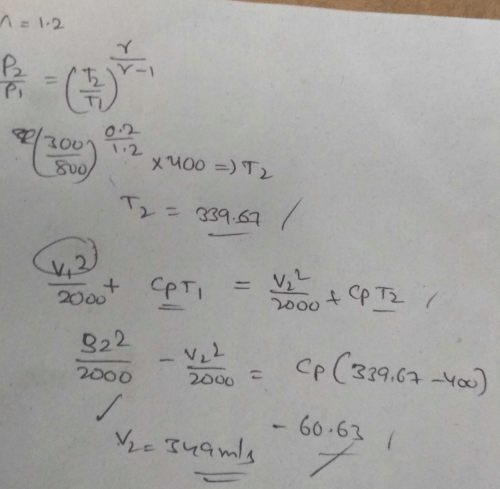
A 5m X 8m X 0.3m size concrete slab ( ρ = 2200 kg/m3, CP = 0.88 kJ/kg-K) is used as thermal storage mass in solar heated house. If slab cools overnight from 230C to 180C in 180C house, what will be the net entropy change associated with process?- a)395.8 kJ/K
- b)265 kJ/K
- c)49.6 kJ/K
- d)3.4 kJ/K
Correct answer is 'D'. Can you explain this answer?
A 5m X 8m X 0.3m size concrete slab ( ρ = 2200 kg/m3, CP = 0.88 kJ/kg-K) is used as thermal storage mass in solar heated house. If slab cools overnight from 230C to 180C in 180C house, what will be the net entropy change associated with process?
a)
395.8 kJ/K
b)
265 kJ/K
c)
49.6 kJ/K
d)
3.4 kJ/K
|
|
Saanvi Mishra answered |
Answer is (d)
The reading of the pressure gauge fitted on a vessel is 25 bar. The atmospheric pressure is 1.03 bar and the value of 'g' is 9.81 m/s2. The absolute pressure in the vessel is
- a)23.97 bar
- b)25 bar
- c)26.03 bar
- d)34.81 bar
Correct answer is option 'C'. Can you explain this answer?
The reading of the pressure gauge fitted on a vessel is 25 bar. The atmospheric pressure is 1.03 bar and the value of 'g' is 9.81 m/s2. The absolute pressure in the vessel is
a)
23.97 bar
b)
25 bar
c)
26.03 bar
d)
34.81 bar
|
|
Neha Joshi answered |
Absolute pressure = gauge pressure + local atmospheric pressure. Gauge pressure can be positive, negative, or zero.
Pabsolute = Pgauge + Patm.
Pgauge = ρgh
Pabsolute = Pgauge + Patm.
Pgauge = ρgh
where,
ρ = Density of liquid.
g = acceleration due to gravity.
h = Height.
Pgauge = 25 bar.
Pgauge = 25 bar.
Patm. = 1.03 bar.
g = 9.8 m/s.
Pabsoltue = 25 + 1.03
Pabsolute = 26.03 bar.
An insulated tank contains 120L of water at 250C ( ρ = 997 kg/m3, CP = 4.18 kJ/kg0C). A 50kg copper block (CP = 0.386 kJ/kg0C) initially at 800C is dropped into water. Total entropy change for this process will be:-- a)3.344 kJ/K
- b)0.204 kJ/K
- c)3.104 kJ/K
- d)6.448 kJ/K
Correct answer is option 'B'. Can you explain this answer?
An insulated tank contains 120L of water at 250C ( ρ = 997 kg/m3, CP = 4.18 kJ/kg0C). A 50kg copper block (CP = 0.386 kJ/kg0C) initially at 800C is dropped into water. Total entropy change for this process will be:-
a)
3.344 kJ/K
b)
0.204 kJ/K
c)
3.104 kJ/K
d)
6.448 kJ/K

|
Sanchita Pillai answered |
The question seems to be incomplete. Please provide the remaining information to answer the question.
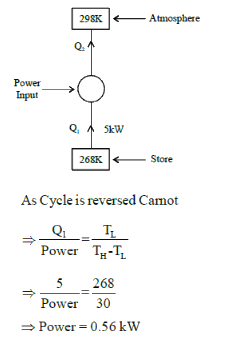
A refrigeration plant for food storage operates as a reversed carnot heat engine cycle. The store is to be maintained at -50C and the heat transfer from the store to the cycle is at the rate of 5 kW. If heat is transferred from the cycle to the atmosphere at a temperature of 250C, the power required to drive the plant is ____kW (Important - Enter only the numerical value in the answer)
Correct answer is between '0.5,0.6'. Can you explain this answer?
A refrigeration plant for food storage operates as a reversed carnot heat engine cycle. The store is to be maintained at -50C and the heat transfer from the store to the cycle is at the rate of 5 kW. If heat is transferred from the cycle to the atmosphere at a temperature of 250C, the power required to drive the plant is ____kW
(Important - Enter only the numerical value in the answer)
|
|
Jaya Menon answered |
Answer is (0.5 - 0.6 kW)
Common data for 6, 7 & 8:
In adiabatic turbine, air enters at 550 kPa, 425K and leaves at 110kPa, 325K. The inlet and exit velocities of air are 150m/s and 50m/s respectivelyT0 = 250C, CP = 1.005 kJ/kg-K, R = 0.287 kJ/kg-KQ. Actual work will be:-- a)170.2 kJ/kg
- b)210 kJ/kg
- c)160 kJ/kg
- d)110.5 kJ/kg
Correct answer is option 'D'. Can you explain this answer?
Common data for 6, 7 & 8:
In adiabatic turbine, air enters at 550 kPa, 425K and leaves at 110kPa, 325K. The inlet and exit velocities of air are 150m/s and 50m/s respectively
In adiabatic turbine, air enters at 550 kPa, 425K and leaves at 110kPa, 325K. The inlet and exit velocities of air are 150m/s and 50m/s respectively
T0 = 250C, CP = 1.005 kJ/kg-K, R = 0.287 kJ/kg-K
Q. Actual work will be:-
a)
170.2 kJ/kg
b)
210 kJ/kg
c)
160 kJ/kg
d)
110.5 kJ/kg
|
|
Avinash Sharma answered |
Answer is (d)

Which of the following is an example of heterogeneous system?- a)Atmospheric air
- b)Mixture of hydrogen and oxygen
- c)Cooling fluid in a radiator
- d)Mixture of ice, water and steam
Correct answer is option 'D'. Can you explain this answer?
Which of the following is an example of heterogeneous system?
a)
Atmospheric air
b)
Mixture of hydrogen and oxygen
c)
Cooling fluid in a radiator
d)
Mixture of ice, water and steam

|
Prerna Menon answered |
Heterogeneous System:
A heterogeneous system refers to a mixture or combination of different substances that are not uniform in composition or properties. In such systems, the individual components can be distinguished and separated from each other by physical means.
Explanation:
Among the given options, the correct example of a heterogeneous system is the mixture of ice, water, and steam (option D). Let's understand why this is the case:
1. Mixture of ice, water, and steam:
- This system consists of three different phases of water: solid ice, liquid water, and gaseous steam.
- Each phase has distinct physical properties, such as density, temperature, and state of matter.
- These components can be visually observed and separated from each other by physical means.
- For example, if we have a container with ice, water, and steam, we can easily separate them by applying heat. The steam will evaporate, the water will remain in liquid form, and the ice will melt into water.
- The components in this system do not have a uniform composition throughout, making it a heterogeneous system.
2. Other options:
- Atmospheric air (option A) is a homogeneous system as it is a mixture of gases (mainly nitrogen, oxygen, carbon dioxide, etc.), which are uniformly distributed throughout the air. The composition of gases remains relatively constant.
- A mixture of hydrogen and oxygen (option B) can be either a homogeneous or heterogeneous system depending on the proportions. If it is a well-mixed mixture, it can be considered homogeneous. However, if the gases are not uniformly mixed, it can be considered heterogeneous.
- Cooling fluid in a radiator (option C) can also be considered a homogeneous system as it typically consists of a well-mixed liquid coolant, such as water or a specialized coolant mixture.
Conclusion:
Among the given options, the mixture of ice, water, and steam (option D) is an example of a heterogeneous system. The components in this system (ice, water, and steam) have different physical properties and can be visually distinguished and separated from each other.
A heterogeneous system refers to a mixture or combination of different substances that are not uniform in composition or properties. In such systems, the individual components can be distinguished and separated from each other by physical means.
Explanation:
Among the given options, the correct example of a heterogeneous system is the mixture of ice, water, and steam (option D). Let's understand why this is the case:
1. Mixture of ice, water, and steam:
- This system consists of three different phases of water: solid ice, liquid water, and gaseous steam.
- Each phase has distinct physical properties, such as density, temperature, and state of matter.
- These components can be visually observed and separated from each other by physical means.
- For example, if we have a container with ice, water, and steam, we can easily separate them by applying heat. The steam will evaporate, the water will remain in liquid form, and the ice will melt into water.
- The components in this system do not have a uniform composition throughout, making it a heterogeneous system.
2. Other options:
- Atmospheric air (option A) is a homogeneous system as it is a mixture of gases (mainly nitrogen, oxygen, carbon dioxide, etc.), which are uniformly distributed throughout the air. The composition of gases remains relatively constant.
- A mixture of hydrogen and oxygen (option B) can be either a homogeneous or heterogeneous system depending on the proportions. If it is a well-mixed mixture, it can be considered homogeneous. However, if the gases are not uniformly mixed, it can be considered heterogeneous.
- Cooling fluid in a radiator (option C) can also be considered a homogeneous system as it typically consists of a well-mixed liquid coolant, such as water or a specialized coolant mixture.
Conclusion:
Among the given options, the mixture of ice, water, and steam (option D) is an example of a heterogeneous system. The components in this system (ice, water, and steam) have different physical properties and can be visually distinguished and separated from each other.
A balloon containing an ideal gas is initially kept in an evacuated and insulated room. The balloon ruptures and the gas fills up the entire room. Which one of the following statement is true at the end of the above process?- a)The internal energy of the gas decreases from its initial value, but the enthalpy remains constant
- b)The internal energy of the gas increases from its initial value, but the enthalpy remains constant
- c)Both internal energy and enthalpy of the gas remains constant
- d)Both internal energy and the enthalpy of the gas increase
Correct answer is option 'C'. Can you explain this answer?
A balloon containing an ideal gas is initially kept in an evacuated and insulated room. The balloon ruptures and the gas fills up the entire room. Which one of the following statement is true at the end of the above process?
a)
The internal energy of the gas decreases from its initial value, but the enthalpy remains constant
b)
The internal energy of the gas increases from its initial value, but the enthalpy remains constant
c)
Both internal energy and enthalpy of the gas remains constant
d)
Both internal energy and the enthalpy of the gas increase

|
Maulik Joshi answered |
Understanding the Scenario
When the balloon containing an ideal gas ruptures in an insulated and evacuated room, the gas expands to fill the available volume. This process involves specific thermodynamic principles that govern the behavior of ideal gases.
Key Principles Involved
- Insulated System: The room is insulated, meaning no heat can enter or leave the system. This leads to adiabatic conditions.
- Ideal Gas Behavior: For an ideal gas, internal energy (U) is primarily a function of temperature (T). Enthalpy (H) is related to internal energy and pressure-volume work.
Internal Energy Changes
- Internal Energy Remains Constant: Since the gas expands into a vacuum (no external work done), and the process is adiabatic, there is no heat transfer. Therefore, the internal energy of the gas does not change.
Enthalpy Considerations
- Enthalpy Remains Constant: Under the conditions of constant temperature and pressure (which is true as the gas expands to fill the room without doing work), the enthalpy also remains unchanged.
Conclusion
In this specific scenario, both internal energy and enthalpy of the gas remain constant after the gas fills the room. Thus, the correct answer is option 'C': Both internal energy and the enthalpy of the gas remain constant.
This is a fundamental outcome of the principles governing ideal gases in adiabatic processes within insulated systems.
When the balloon containing an ideal gas ruptures in an insulated and evacuated room, the gas expands to fill the available volume. This process involves specific thermodynamic principles that govern the behavior of ideal gases.
Key Principles Involved
- Insulated System: The room is insulated, meaning no heat can enter or leave the system. This leads to adiabatic conditions.
- Ideal Gas Behavior: For an ideal gas, internal energy (U) is primarily a function of temperature (T). Enthalpy (H) is related to internal energy and pressure-volume work.
Internal Energy Changes
- Internal Energy Remains Constant: Since the gas expands into a vacuum (no external work done), and the process is adiabatic, there is no heat transfer. Therefore, the internal energy of the gas does not change.
Enthalpy Considerations
- Enthalpy Remains Constant: Under the conditions of constant temperature and pressure (which is true as the gas expands to fill the room without doing work), the enthalpy also remains unchanged.
Conclusion
In this specific scenario, both internal energy and enthalpy of the gas remain constant after the gas fills the room. Thus, the correct answer is option 'C': Both internal energy and the enthalpy of the gas remain constant.
This is a fundamental outcome of the principles governing ideal gases in adiabatic processes within insulated systems.
Consider the following:
1. Temperature
2. Viscosity
3. Specific entropy
4. Thermal conductivityWhich of the above properties of a system is/are intensive- a)1 only
- b)2 and 3 only
- c)2, 3 and 4 only
- d)1 and 2 only
Correct answer is option 'D'. Can you explain this answer?
Consider the following:
1. Temperature
2. Viscosity
3. Specific entropy
4. Thermal conductivity
1. Temperature
2. Viscosity
3. Specific entropy
4. Thermal conductivity
Which of the above properties of a system is/are intensive
a)
1 only
b)
2 and 3 only
c)
2, 3 and 4 only
d)
1 and 2 only
|
|
Amrita Chauhan answered |
Intensive and Extensive Properties:
In thermodynamics, properties of a system can be classified as either intensive or extensive. Intensive properties are independent of the size or amount of the system, while extensive properties depend on the size or amount of the system.
Explanation of the given properties:
1. Entropy:
Entropy is a measure of the disorder or randomness of a system. It is a state function and is independent of the size or amount of the system. Therefore, entropy is an intensive property.
2. Viscosity:
Viscosity is a measure of a fluid's resistance to flow. It is determined by the internal friction between the fluid molecules. Viscosity is independent of the size or amount of the fluid and is therefore an intensive property.
3. Temperature:
Temperature is a measure of the average kinetic energy of the particles in a system. It is independent of the size or amount of the system and is therefore an intensive property.
4. Specific heat at constant volume:
Specific heat at constant volume is the amount of heat required to raise the temperature of a unit mass of a substance by one degree Celsius at constant volume. It is a material-specific property and is independent of the size or amount of the substance. Therefore, specific heat at constant volume is an intensive property.
Conclusion:
From the explanations above, we can conclude that options 'a', 'b', 'c' are incorrect as they do not include all the intensive properties. Option 'D' is the correct answer as it includes all the given properties (viscosity, temperature, and specific heat at constant volume) that are intensive properties.
In thermodynamics, properties of a system can be classified as either intensive or extensive. Intensive properties are independent of the size or amount of the system, while extensive properties depend on the size or amount of the system.
Explanation of the given properties:
1. Entropy:
Entropy is a measure of the disorder or randomness of a system. It is a state function and is independent of the size or amount of the system. Therefore, entropy is an intensive property.
2. Viscosity:
Viscosity is a measure of a fluid's resistance to flow. It is determined by the internal friction between the fluid molecules. Viscosity is independent of the size or amount of the fluid and is therefore an intensive property.
3. Temperature:
Temperature is a measure of the average kinetic energy of the particles in a system. It is independent of the size or amount of the system and is therefore an intensive property.
4. Specific heat at constant volume:
Specific heat at constant volume is the amount of heat required to raise the temperature of a unit mass of a substance by one degree Celsius at constant volume. It is a material-specific property and is independent of the size or amount of the substance. Therefore, specific heat at constant volume is an intensive property.
Conclusion:
From the explanations above, we can conclude that options 'a', 'b', 'c' are incorrect as they do not include all the intensive properties. Option 'D' is the correct answer as it includes all the given properties (viscosity, temperature, and specific heat at constant volume) that are intensive properties.
A diathermic wall is one which- a)prevents thermal interaction
- b)permits thermal interaction
- c)encourages thermal interaction
- d)discourages thermal interaction
Correct answer is option 'B'. Can you explain this answer?
A diathermic wall is one which
a)
prevents thermal interaction
b)
permits thermal interaction
c)
encourages thermal interaction
d)
discourages thermal interaction

|
Jay Menon answered |
Diathermic Wall in Thermal Interaction
Definition: A diathermic wall is a type of wall that permits thermal interaction between two or more systems.
Explanation:
Thermal interaction refers to the exchange of thermal energy between two or more systems. A diathermic wall is a type of wall that allows this exchange of thermal energy to occur. The wall is said to be diathermic because it is permeable to heat transfer. In other words, heat can flow through the wall and be exchanged between the systems on either side of the wall.
A diathermic wall is commonly used in many engineering applications where thermal interaction between different systems is required. For instance, in a heat exchanger, a diathermic wall separates the two fluids that are being exchanged. The wall allows heat to flow between the fluids, thus facilitating the transfer of thermal energy from one fluid to the other.
Examples:
Some common examples of diathermic walls include:
- The walls of a furnace that allow heat to pass from the furnace to the surrounding environment.
- The walls of a heat exchanger that allow thermal energy to transfer between two or more fluids.
- The walls of a building that allow heat to flow from the inside to the outside or vice versa.
- The walls of a refrigerator that allow heat to flow from the inside to the outside.
Conclusion:
In summary, a diathermic wall is a type of wall that permits thermal interaction between two or more systems. The wall allows heat to flow through it and be exchanged between the systems on either side of the wall. Diathermic walls are commonly used in many engineering applications where thermal interaction is required, such as in heat exchangers, furnaces, and buildings.
Definition: A diathermic wall is a type of wall that permits thermal interaction between two or more systems.
Explanation:
Thermal interaction refers to the exchange of thermal energy between two or more systems. A diathermic wall is a type of wall that allows this exchange of thermal energy to occur. The wall is said to be diathermic because it is permeable to heat transfer. In other words, heat can flow through the wall and be exchanged between the systems on either side of the wall.
A diathermic wall is commonly used in many engineering applications where thermal interaction between different systems is required. For instance, in a heat exchanger, a diathermic wall separates the two fluids that are being exchanged. The wall allows heat to flow between the fluids, thus facilitating the transfer of thermal energy from one fluid to the other.
Examples:
Some common examples of diathermic walls include:
- The walls of a furnace that allow heat to pass from the furnace to the surrounding environment.
- The walls of a heat exchanger that allow thermal energy to transfer between two or more fluids.
- The walls of a building that allow heat to flow from the inside to the outside or vice versa.
- The walls of a refrigerator that allow heat to flow from the inside to the outside.
Conclusion:
In summary, a diathermic wall is a type of wall that permits thermal interaction between two or more systems. The wall allows heat to flow through it and be exchanged between the systems on either side of the wall. Diathermic walls are commonly used in many engineering applications where thermal interaction is required, such as in heat exchangers, furnaces, and buildings.
Air enters heat exchanger at 95 kPa and 200C with rate of 1.6 m3/s. The combustion gases (CP = 1.1 kJ/kg-0C) enters at 1800C with rate of 2.2 kg/s and leave at 950C. Rate of entropy generation is- a)0.455 kW/K
- b)0.91 kW/K
- c)9.1 kW/K
- d)0.091 kW/K
Correct answer is option 'D'. Can you explain this answer?
Air enters heat exchanger at 95 kPa and 200C with rate of 1.6 m3/s. The combustion gases (CP = 1.1 kJ/kg-0C) enters at 1800C with rate of 2.2 kg/s and leave at 950C. Rate of entropy generation is
a)
0.455 kW/K
b)
0.91 kW/K
c)
9.1 kW/K
d)
0.091 kW/K
|
|
Saikat Choudhary answered |
To calculate the rate of entropy generation, we can use the following equation:
Rate of entropy generation = mass flow rate of air * entropy change of air + mass flow rate of combustion gases * entropy change of combustion gases
1. Entropy change of air:
The entropy change of air can be calculated using the equation:
ΔS_air = C_p * ln(T2/T1)
where ΔS_air is the entropy change of air, C_p is the specific heat capacity of air at constant pressure, T1 is the initial temperature of the air, and T2 is the final temperature of the air.
Given:
C_p = 1.1 kJ/kg-°C
T1 = 200°C
T2 = 950°C
Converting the temperature to Kelvin:
T1 = 200 + 273 = 473 K
T2 = 950 + 273 = 1223 K
Using the equation, we can calculate the entropy change of air:
ΔS_air = 1.1 * ln(1223/473) kJ/kg = 1.1 * ln(2.587) kJ/kg
2. Entropy change of combustion gases:
The entropy change of combustion gases can also be calculated using the same equation:
ΔS_combustion gases = C_p * ln(T4/T3)
where ΔS_combustion gases is the entropy change of combustion gases, C_p is the specific heat capacity of combustion gases at constant pressure, T3 is the initial temperature of the combustion gases, and T4 is the final temperature of the combustion gases.
Given:
C_p = 1.1 kJ/kg-°C
T3 = 1800°C
T4 = 950°C
Converting the temperature to Kelvin:
T3 = 1800 + 273 = 2073 K
T4 = 950 + 273 = 1223 K
Using the equation, we can calculate the entropy change of combustion gases:
ΔS_combustion gases = 1.1 * ln(1223/2073) kJ/kg = 1.1 * ln(0.591) kJ/kg
3. Rate of entropy generation:
Lastly, we can calculate the rate of entropy generation using the equation:
Rate of entropy generation = mass flow rate of air * entropy change of air + mass flow rate of combustion gases * entropy change of combustion gases
Given:
Mass flow rate of air = 1.6 m^3/s
Mass flow rate of combustion gases = 2.2 kg/s
Substituting the values into the equation:
Rate of entropy generation = 1.6 * ΔS_air + 2.2 * ΔS_combustion gases
= 1.6 * (1.1 * ln(2.587)) + 2.2 * (1.1 * ln(0.591)) kJ/s
= 0.091 kW/K
Therefore, the correct answer is option D: 0.091 kW/K.
Rate of entropy generation = mass flow rate of air * entropy change of air + mass flow rate of combustion gases * entropy change of combustion gases
1. Entropy change of air:
The entropy change of air can be calculated using the equation:
ΔS_air = C_p * ln(T2/T1)
where ΔS_air is the entropy change of air, C_p is the specific heat capacity of air at constant pressure, T1 is the initial temperature of the air, and T2 is the final temperature of the air.
Given:
C_p = 1.1 kJ/kg-°C
T1 = 200°C
T2 = 950°C
Converting the temperature to Kelvin:
T1 = 200 + 273 = 473 K
T2 = 950 + 273 = 1223 K
Using the equation, we can calculate the entropy change of air:
ΔS_air = 1.1 * ln(1223/473) kJ/kg = 1.1 * ln(2.587) kJ/kg
2. Entropy change of combustion gases:
The entropy change of combustion gases can also be calculated using the same equation:
ΔS_combustion gases = C_p * ln(T4/T3)
where ΔS_combustion gases is the entropy change of combustion gases, C_p is the specific heat capacity of combustion gases at constant pressure, T3 is the initial temperature of the combustion gases, and T4 is the final temperature of the combustion gases.
Given:
C_p = 1.1 kJ/kg-°C
T3 = 1800°C
T4 = 950°C
Converting the temperature to Kelvin:
T3 = 1800 + 273 = 2073 K
T4 = 950 + 273 = 1223 K
Using the equation, we can calculate the entropy change of combustion gases:
ΔS_combustion gases = 1.1 * ln(1223/2073) kJ/kg = 1.1 * ln(0.591) kJ/kg
3. Rate of entropy generation:
Lastly, we can calculate the rate of entropy generation using the equation:
Rate of entropy generation = mass flow rate of air * entropy change of air + mass flow rate of combustion gases * entropy change of combustion gases
Given:
Mass flow rate of air = 1.6 m^3/s
Mass flow rate of combustion gases = 2.2 kg/s
Substituting the values into the equation:
Rate of entropy generation = 1.6 * ΔS_air + 2.2 * ΔS_combustion gases
= 1.6 * (1.1 * ln(2.587)) + 2.2 * (1.1 * ln(0.591)) kJ/s
= 0.091 kW/K
Therefore, the correct answer is option D: 0.091 kW/K.
A 5m X 8m X 0.3m size concrete slab ( ρ = 2200 kg/m3, CP = 0.88 kJ/kg-K) is used as thermal storage mass in solar heated house. If slab cools overnight from 230C to 180C in 180C house, what will be the net entropy change associated with process?- a)395.8 kJ/K
- b)265 kJ/K
- c)49.6 kJ/K
- d)3.4 kJ/K
Correct answer is option 'D'. Can you explain this answer?
A 5m X 8m X 0.3m size concrete slab ( ρ = 2200 kg/m3, CP = 0.88 kJ/kg-K) is used as thermal storage mass in solar heated house. If slab cools overnight from 230C to 180C in 180C house, what will be the net entropy change associated with process?
a)
395.8 kJ/K
b)
265 kJ/K
c)
49.6 kJ/K
d)
3.4 kJ/K

|
Sandeep Sen answered |
Answer is (d)
For an isolated system executing a process
1. no heat transfer takes place
2. no work is done
3. no mass crosses the boundary
4. no chemical reaction takes place within the systemWhich of the above statement are correct?- a)1,2 and 3
- b)1,3 and 4
- c)2, 3 and 4
- d)all of the above
Correct answer is option 'A'. Can you explain this answer?
For an isolated system executing a process
1. no heat transfer takes place
2. no work is done
3. no mass crosses the boundary
4. no chemical reaction takes place within the system
1. no heat transfer takes place
2. no work is done
3. no mass crosses the boundary
4. no chemical reaction takes place within the system
Which of the above statement are correct?
a)
1,2 and 3
b)
1,3 and 4
c)
2, 3 and 4
d)
all of the above
|
|
Gaurav Kapoor answered |
Isolated System and its characteristics
An isolated system is a thermodynamic system that does not exchange energy or matter with its surroundings. The system is completely self-contained, and there is no interaction with the environment. The system can be a physical or theoretical construct, and it is often used in thermodynamics to simplify the analysis of a process or system.
Characteristics of an isolated system:
- No heat transfer takes place: In an isolated system, there is no transfer of heat energy between the system and its surroundings. The system is thermally insulated, and the temperature of the system remains constant.
- No work is done: An isolated system does not perform any work on its surroundings, nor is any work done on the system by the surroundings.
- No mass crosses the boundary: In an isolated system, there is no transfer of matter across the system boundary. The total mass of the system remains constant.
- No chemical reaction takes place within the system: An isolated system does not undergo any chemical reactions within the system.
Correct statement for an isolated system
From the given options, the correct statement for an isolated system is:
a) 1, 2, and 3
Explanation:
- No heat transfer takes place: As an isolated system is thermally insulated, there is no transfer of heat between the system and its surroundings.
- No work is done: An isolated system does not perform any work on its surroundings or vice versa. Hence, no work is done.
- No mass crosses the boundary: In an isolated system, there is no transfer of matter across the system boundary. Hence, the total mass of the system remains constant.
Therefore, option 'a' is the correct statement for an isolated system.
An isolated system is a thermodynamic system that does not exchange energy or matter with its surroundings. The system is completely self-contained, and there is no interaction with the environment. The system can be a physical or theoretical construct, and it is often used in thermodynamics to simplify the analysis of a process or system.
Characteristics of an isolated system:
- No heat transfer takes place: In an isolated system, there is no transfer of heat energy between the system and its surroundings. The system is thermally insulated, and the temperature of the system remains constant.
- No work is done: An isolated system does not perform any work on its surroundings, nor is any work done on the system by the surroundings.
- No mass crosses the boundary: In an isolated system, there is no transfer of matter across the system boundary. The total mass of the system remains constant.
- No chemical reaction takes place within the system: An isolated system does not undergo any chemical reactions within the system.
Correct statement for an isolated system
From the given options, the correct statement for an isolated system is:
a) 1, 2, and 3
Explanation:
- No heat transfer takes place: As an isolated system is thermally insulated, there is no transfer of heat between the system and its surroundings.
- No work is done: An isolated system does not perform any work on its surroundings or vice versa. Hence, no work is done.
- No mass crosses the boundary: In an isolated system, there is no transfer of matter across the system boundary. Hence, the total mass of the system remains constant.
Therefore, option 'a' is the correct statement for an isolated system.
Zeroth law of thermodynamics states that:- a)two thermodynamic systems are always in thermal equilibrium with each other.
- b)if two systems are in thermal equilibrium, then the third system will also be in thermal equilibrium.
- c)two systems not in thermal equilibrium with a third system are also not in thermal equilibrium with each other.
- d)when two systems are in thermal equilibrium with a third system, they are in thermal equilibrium with each other.
Correct answer is option 'D'. Can you explain this answer?
Zeroth law of thermodynamics states that:
a)
two thermodynamic systems are always in thermal equilibrium with each other.
b)
if two systems are in thermal equilibrium, then the third system will also be in thermal equilibrium.
c)
two systems not in thermal equilibrium with a third system are also not in thermal equilibrium with each other.
d)
when two systems are in thermal equilibrium with a third system, they are in thermal equilibrium with each other.
|
|
Zoya Sharma answered |
The zeroth law of thermodynamics states that if two bodies are each in thermal equilibrium with some third body, then they are also in equilibrium with each other. ... This says in essence that the three bodies are all the same temperature.
Work is done on a adiabatic system due to which its velocity changes from 10 m/s to 20 m/s, elevation
increases by 20 m and temperature increases by 1 K. The mass of the system is 10 kg, Cv = 100 J/(kg.K) and gravitational acceleration is 10 m/s2. If there is no change in any other component of the energy of the system, the magnitude of total work done (in kJ) on the system is Correct answer is '4.5'. Can you explain this answer?
Work is done on a adiabatic system due to which its velocity changes from 10 m/s to 20 m/s, elevation
increases by 20 m and temperature increases by 1 K. The mass of the system is 10 kg, Cv = 100 J/(kg.K) and gravitational acceleration is 10 m/s2. If there is no change in any other component of the energy of the system, the magnitude of total work done (in kJ) on the system is
increases by 20 m and temperature increases by 1 K. The mass of the system is 10 kg, Cv = 100 J/(kg.K) and gravitational acceleration is 10 m/s2. If there is no change in any other component of the energy of the system, the magnitude of total work done (in kJ) on the system is

|
Pk Academy answered |
Given: C1 = 10 m/s, C2 = 20 m/s, z2 – z1 = 20 m,
T2 – T1 = 1 K
CV = 100 J/kg-K, g = 10 m/s2, δQ = 0
T2 – T1 = 1 K
CV = 100 J/kg-K, g = 10 m/s2, δQ = 0
Assuming compressible fluid with γ = 1

SFEE:
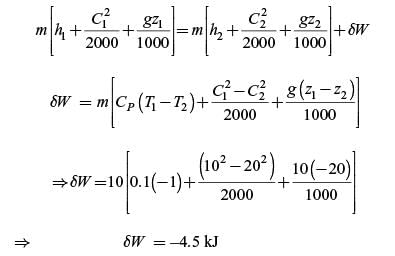
On the system, δ W = 4.5 kJ
Hence, the correct answer is 4.5.
A 50-Kg block of iron casting at 500K is thrown into a large lake that is at a temperature of 285K. The iron block eventually reaches thermal equilibrium with the lake water. Assuming an average specific heat of 0.45 kJ/Kg-K for the iron, the entropy generation during the process is:- a)12.65 kJ/K
- b)16.97 kJ/K
- c)4.32 kJ/K
- d)29.62 kJ/K
Correct answer is option 'C'. Can you explain this answer?
A 50-Kg block of iron casting at 500K is thrown into a large lake that is at a temperature of 285K. The iron block eventually reaches thermal equilibrium with the lake water. Assuming an average specific heat of 0.45 kJ/Kg-K for the iron, the entropy generation during the process is:
a)
12.65 kJ/K
b)
16.97 kJ/K
c)
4.32 kJ/K
d)
29.62 kJ/K
|
|
Aditya Deshmukh answered |
Specific heat of iron block:
Cv = m cv = 22.5 kJ/K
Entropy of iron:
S(T) = ∫dS = ∫ 1/T CvdT = Cv lnT
Entropy change of iron:
∆Si = Cv ln(To/T) = -12.65 kJ/K
Entropy change of water:
∆Sw = ∆Q/To = Cv(T/To -1) = +16.97 kJ/K
Total entropy change:
∆S = ∆SI + ∆Sw = +4.3 kJ/K
In a quasiequilibrium process, the pressure in a system
- a)unconstant
- b)varies with temperature
- c)constant at any given instant throughout the system
- d)increase if volume increases
Correct answer is option 'C'. Can you explain this answer?
In a quasiequilibrium process, the pressure in a system
a)
unconstant
b)
varies with temperature
c)
constant at any given instant throughout the system
d)
increase if volume increases
|
|
Meera Bose answered |
Explanation:
Quasiequilibrium process is a process that occurs slowly enough for the system to remain in thermal and mechanical equilibrium at all times. In such a process, the pressure within the system is everywhere constant at an instant. This is because any variation in pressure within the system would cause the system to move towards a state of equilibrium, which is characterized by uniform pressure throughout the system. Therefore, in a quasiequilibrium process, the pressure remains constant throughout the system at any given instant.
Some important points to note about quasiequilibrium process are:
- It is a reversible process, meaning that it can be reversed by an infinitesimal change in the external conditions.
- It is a slow process, meaning that it occurs over a long period of time, allowing the system to remain in thermal and mechanical equilibrium at all times.
- It is an idealized process, meaning that it is not possible to achieve it in practice, but it provides a useful theoretical framework for analyzing real-world processes.
Conclusion:
In conclusion, in a quasiequilibrium process, the pressure within the system remains constant at any given instant. This is because the system is always in thermal and mechanical equilibrium, and any variation in pressure would cause the system to move towards a state of equilibrium characterized by uniform pressure throughout the system.
Quasiequilibrium process is a process that occurs slowly enough for the system to remain in thermal and mechanical equilibrium at all times. In such a process, the pressure within the system is everywhere constant at an instant. This is because any variation in pressure within the system would cause the system to move towards a state of equilibrium, which is characterized by uniform pressure throughout the system. Therefore, in a quasiequilibrium process, the pressure remains constant throughout the system at any given instant.
Some important points to note about quasiequilibrium process are:
- It is a reversible process, meaning that it can be reversed by an infinitesimal change in the external conditions.
- It is a slow process, meaning that it occurs over a long period of time, allowing the system to remain in thermal and mechanical equilibrium at all times.
- It is an idealized process, meaning that it is not possible to achieve it in practice, but it provides a useful theoretical framework for analyzing real-world processes.
Conclusion:
In conclusion, in a quasiequilibrium process, the pressure within the system remains constant at any given instant. This is because the system is always in thermal and mechanical equilibrium, and any variation in pressure would cause the system to move towards a state of equilibrium characterized by uniform pressure throughout the system.
A football was inflated to a gauge pressure of 1 bar when the ambient temperature was 15°C. When the game started next day, the air temperature at the stadium was 5°C. Assume that the volume of the football remains constant at 2500 cm3. Gauge pressure of air to which the ball must have been originally inflated so that it would equal 1 bar gauge at the stadium is _____.- a)2.23 bar
- b)1.94 bar
- c)1.07 bar
- d)1.00 bar
Correct answer is option 'C'. Can you explain this answer?
A football was inflated to a gauge pressure of 1 bar when the ambient temperature was 15°C. When the game started next day, the air temperature at the stadium was 5°C. Assume that the volume of the football remains constant at 2500 cm3. Gauge pressure of air to which the ball must have been originally inflated so that it would equal 1 bar gauge at the stadium is _____.
a)
2.23 bar
b)
1.94 bar
c)
1.07 bar
d)
1.00 bar

|
Manasa Sen answered |
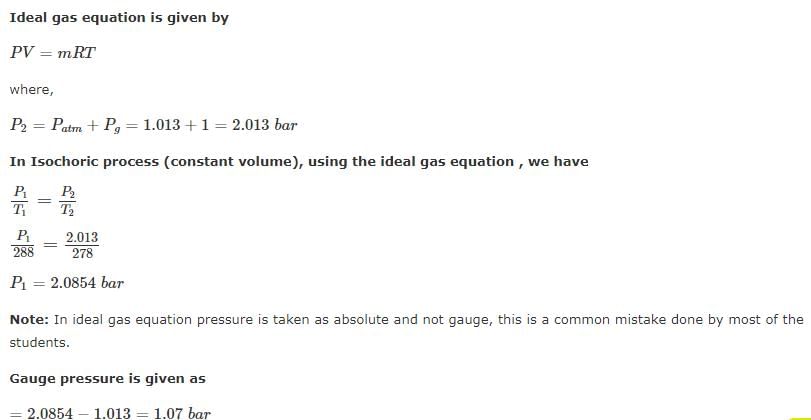
Air at 100 KPa and 280 K is compressed steadily to 600 KPa and 400 K. The mass flow rate of air is 0.02 Kg/s and a heat loss of 16 kJ/Kg occurs during the process. The power input to compressor will be_____kW
Correct answer is between '-2.730,-2.372'. Can you explain this answer?
Air at 100 KPa and 280 K is compressed steadily to 600 KPa and 400 K. The mass flow rate of air is 0.02 Kg/s and a heat loss of 16 kJ/Kg occurs during the process. The power input to compressor will be_____kW

|
Manasa Bose answered |
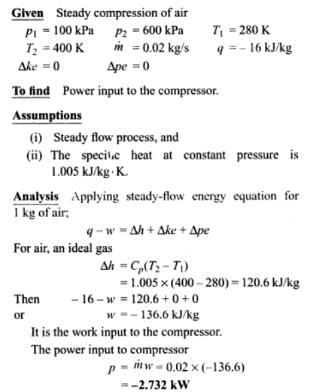
According to thermodynamics, the universe is defined as_______.
- a)System
- b)System + Surrounding
- c)Surrounding
- d)None of these
Correct answer is option 'B'. Can you explain this answer?
According to thermodynamics, the universe is defined as_______.
a)
System
b)
System + Surrounding
c)
Surrounding
d)
None of these

|
Anjana Mukherjee answered |
In thermodynamics, a system is any part of the universe which is under observation and the rest of the universe, except the system, are called its surroundings.
So, universe = system + surroundings.
Common data for 20 & 21:An insulated nozzle is there in which helium gas enters with velocity of 10 m/s, operating at steady state at 1300K, 4 bar. At the exit, temperature & pressure of the helium are 900K and 1.45bar, respectively. The environment is at T0 = 200C and P0 = 1 atm.
Take CP = 5.1926 kJ/kg-K, R = 2.0769 kJ/kg-K, γ = 1.667 Q. Exit velocity of nozzle is: -- a)1962 m/s
- b)98 m/s
- c)64 m/s
- d)2038 m/s
Correct answer is option 'D'. Can you explain this answer?
Common data for 20 & 21:
An insulated nozzle is there in which helium gas enters with velocity of 10 m/s, operating at steady state at 1300K, 4 bar. At the exit, temperature & pressure of the helium are 900K and 1.45bar, respectively. The environment is at T0 = 200C and P0 = 1 atm.
Take CP = 5.1926 kJ/kg-K, R = 2.0769 kJ/kg-K, γ = 1.667
Take CP = 5.1926 kJ/kg-K, R = 2.0769 kJ/kg-K, γ = 1.667
Q. Exit velocity of nozzle is: -
a)
1962 m/s
b)
98 m/s
c)
64 m/s
d)
2038 m/s

|
Saikat Gupta answered |
Answer is (d)
The study of thermodynamics provides answer to the followings:
1. whether a process is feasible or not
2. to quantity the energy required for a process
3. rate or speed with which a process occurs
4. extent to which a reaction/process takes place
Which of the above statements are correct?
- a)1,2 and 3
- b)1 and 2
- c)1,2 and 4
- d)2, 3 and 4
Correct answer is option 'C'. Can you explain this answer?
The study of thermodynamics provides answer to the followings:
1. whether a process is feasible or not
2. to quantity the energy required for a process
3. rate or speed with which a process occurs
4. extent to which a reaction/process takes place
1. whether a process is feasible or not
2. to quantity the energy required for a process
3. rate or speed with which a process occurs
4. extent to which a reaction/process takes place
Which of the above statements are correct?
a)
1,2 and 3
b)
1 and 2
c)
1,2 and 4
d)
2, 3 and 4
|
|
Ruchi Ahuja answered |
Thermodynamics is a branch of physics that deals with the study of energy and its transformation. It has various applications in engineering, chemistry, and other fields. The study of thermodynamics provides answers to many questions related to energy and its transformation. Let's discuss each of the given statements in detail:
1. Feasibility of a process:
Thermodynamics helps in determining whether a process is feasible or not. Feasibility refers to whether the process can occur or not, given the conditions such as temperature, pressure, and other parameters. For example, if a chemical reaction requires high temperature and pressure to occur, thermodynamics can help determine whether it is feasible or not under the given conditions.
2. Energy required for a process:
Thermodynamics helps in quantifying the energy required for a process. Energy is required for any process to occur, and thermodynamics helps in determining how much energy is required. For example, in a steam turbine, thermodynamics helps in determining the amount of energy required to produce steam and the amount of energy produced by the turbine.
3. Rate or speed of a process:
Thermodynamics also helps in determining the rate or speed with which a process occurs. The rate of a process refers to how fast or slow it occurs. For example, in a chemical reaction, thermodynamics can help determine how fast the reactants are converted into products.
4. Extent of a reaction/process:
Thermodynamics helps in determining the extent to which a reaction or process takes place. The extent refers to how much of the reactants are converted into products. For example, in a combustion reaction, thermodynamics can help determine how much of the fuel is converted into energy.
Conclusion:
In summary, thermodynamics is a very important field of study that provides answers to many questions related to energy and its transformation. It helps in determining the feasibility of a process, quantifying the energy required, determining the rate of a process, and determining the extent of a reaction or process.
1. Feasibility of a process:
Thermodynamics helps in determining whether a process is feasible or not. Feasibility refers to whether the process can occur or not, given the conditions such as temperature, pressure, and other parameters. For example, if a chemical reaction requires high temperature and pressure to occur, thermodynamics can help determine whether it is feasible or not under the given conditions.
2. Energy required for a process:
Thermodynamics helps in quantifying the energy required for a process. Energy is required for any process to occur, and thermodynamics helps in determining how much energy is required. For example, in a steam turbine, thermodynamics helps in determining the amount of energy required to produce steam and the amount of energy produced by the turbine.
3. Rate or speed of a process:
Thermodynamics also helps in determining the rate or speed with which a process occurs. The rate of a process refers to how fast or slow it occurs. For example, in a chemical reaction, thermodynamics can help determine how fast the reactants are converted into products.
4. Extent of a reaction/process:
Thermodynamics helps in determining the extent to which a reaction or process takes place. The extent refers to how much of the reactants are converted into products. For example, in a combustion reaction, thermodynamics can help determine how much of the fuel is converted into energy.
Conclusion:
In summary, thermodynamics is a very important field of study that provides answers to many questions related to energy and its transformation. It helps in determining the feasibility of a process, quantifying the energy required, determining the rate of a process, and determining the extent of a reaction or process.
A small steam whistle (perfectly insulated and doing no shaft work) causes a drop of 0.8 kJ/kg in enthalpy of steam from entry to exit. The kinetic energy of the steam at entry is negligible, the velocity of steam at exit is- a)4 m/s
- b)40 m/s
- c)80 m/s
- d)120 m/s
Correct answer is option 'B'. Can you explain this answer?
A small steam whistle (perfectly insulated and doing no shaft work) causes a drop of 0.8 kJ/kg in enthalpy of steam from entry to exit. The kinetic energy of the steam at entry is negligible, the velocity of steam at exit is
a)
4 m/s
b)
40 m/s
c)
80 m/s
d)
120 m/s

|
Gate Funda answered |
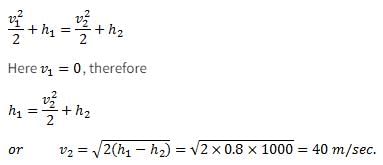
Common data for 6, 7 & 8:
In adiabatic turbine, air enters at 550 kPa, 425K and leaves at 110kPa, 325K. The inlet and exit velocities of air are 150m/s and 50m/s respectivelyT0 = 250C, CP = 1.005 kJ/kg-K, R = 0.287 kJ/kg-K Q. Reversible work will be:-- a)196 kJ/kg
- b)166.8 kJ/kg
- c)232 kJ/kg
- d)311 kJ/kg
Correct answer is option 'B'. Can you explain this answer?
Common data for 6, 7 & 8:
In adiabatic turbine, air enters at 550 kPa, 425K and leaves at 110kPa, 325K. The inlet and exit velocities of air are 150m/s and 50m/s respectively
In adiabatic turbine, air enters at 550 kPa, 425K and leaves at 110kPa, 325K. The inlet and exit velocities of air are 150m/s and 50m/s respectively
T0 = 250C, CP = 1.005 kJ/kg-K, R = 0.287 kJ/kg-K
Q. Reversible work will be:-
a)
196 kJ/kg
b)
166.8 kJ/kg
c)
232 kJ/kg
d)
311 kJ/kg

|
Abhay Kapoor answered |
The common data for 6 and 7 can include the following:
- Both numbers are positive integers.
- They are consecutive numbers, with 6 being the smaller number and 7 being the larger number.
- Both numbers are prime numbers, as they are only divisible by 1 and themselves.
- They are consecutive odd numbers, as they are not divisible by 2.
- They are consecutive natural numbers, as they are both counting numbers.
- Both numbers are single-digit numbers.
- Both numbers are positive integers.
- They are consecutive numbers, with 6 being the smaller number and 7 being the larger number.
- Both numbers are prime numbers, as they are only divisible by 1 and themselves.
- They are consecutive odd numbers, as they are not divisible by 2.
- They are consecutive natural numbers, as they are both counting numbers.
- Both numbers are single-digit numbers.
Which one ofthe following is extensive property of a thermodynamics system- a)Volume
- b)Pressure
- c)Temperature
- d)Density
Correct answer is option 'A'. Can you explain this answer?
Which one ofthe following is extensive property of a thermodynamics system
a)
Volume
b)
Pressure
c)
Temperature
d)
Density

|
Kirti Sharma answered |
Since volume depends on mass hence it is extensive property.
The sequence of processes that eventually returns the working substance to its original state is known as- a)event
- b)process
- c)thermodynamic property
- d)thermodynamic cycle
Correct answer is option 'D'. Can you explain this answer?
The sequence of processes that eventually returns the working substance to its original state is known as
a)
event
b)
process
c)
thermodynamic property
d)
thermodynamic cycle

|
Hiral Sharma answered |
Thermodynamic cycle can be defined as a series of state changes such that the final and initial state is identical.
Which of the following quantities is not the property of the system- a)Pressure
- b)Temperature
- c)Density
- d)Heat
Correct answer is option 'D'. Can you explain this answer?
Which of the following quantities is not the property of the system
a)
Pressure
b)
Temperature
c)
Density
d)
Heat

|
Devika Tiwari answered |
Since heat transfer is the path function hence it is not the property of the system.
Match the following List-I with List-ll:List-I
A. Centrifugal fan
B. Control volume
C. Intensive property
D. Microscopic propertyList-ll
1. Open system
2. Internal energy
3. Filling a tire at air station
4. Specific energy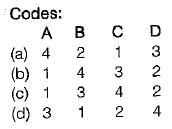
- a)a
- b)b
- c)c
- d)d
Correct answer is option 'C'. Can you explain this answer?
Match the following List-I with List-ll:
List-I
A. Centrifugal fan
B. Control volume
C. Intensive property
D. Microscopic property
A. Centrifugal fan
B. Control volume
C. Intensive property
D. Microscopic property
List-ll
1. Open system
2. Internal energy
3. Filling a tire at air station
4. Specific energy
1. Open system
2. Internal energy
3. Filling a tire at air station
4. Specific energy

a)
a
b)
b
c)
c
d)
d

|
Bidhan Das answered |
Control volume is also known as open system
Convert the following readings of pressure to kPa, assuming that the barometer reads 760mm of Hg and match the List-I and List-II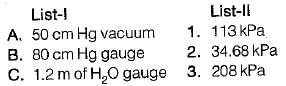

- a)a
- b)b
- c)c
- d)d
Correct answer is option 'C'. Can you explain this answer?
Convert the following readings of pressure to kPa, assuming that the barometer reads 760mm of Hg and match the List-I and List-II
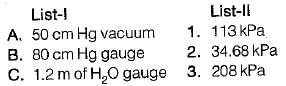

a)
a
b)
b
c)
c
d)
d

|
Harsh Khanna answered |
50 cm Hg vacuume:

80 cm Hg gauge:
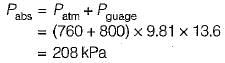
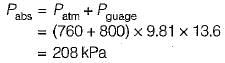
1.2 m of H20 guage:
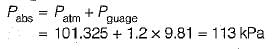
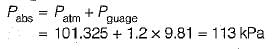
For a system to be in thermodynamic equilibrium the system and its surroundings are to be in- a)Thermal equilibrium
- b)Chemical equilibrium
- c)Mechanical equilibrium
- d)Thermal, chemical and mechanical equilibrium
Correct answer is option 'D'. Can you explain this answer?
For a system to be in thermodynamic equilibrium the system and its surroundings are to be in
a)
Thermal equilibrium
b)
Chemical equilibrium
c)
Mechanical equilibrium
d)
Thermal, chemical and mechanical equilibrium
|
|
Ashish Pillai answered |
A system said to be in thermodynamic equilibrium with surrounding if:
It is in thermal, chemical as well as mechanical equilibrium with the surrounding.
And when system is in thermodynamic equilibrium there won't be any spontaneous change.
A closed thermodynamic system is one in which- a)there is no energy or mass transfer across the boundary
- b)there is no mass transfer, but energy transfer exists
- c)there is no energy transfer, but mass transfer exists
- d)both energy and mass transfer takes place across the boundary but the mass transfer is controlled by valves
Correct answer is option 'B'. Can you explain this answer?
A closed thermodynamic system is one in which
a)
there is no energy or mass transfer across the boundary
b)
there is no mass transfer, but energy transfer exists
c)
there is no energy transfer, but mass transfer exists
d)
both energy and mass transfer takes place across the boundary but the mass transfer is controlled by valves

|
Subham Unni answered |
A closed thermodynamic system is one in which there is no mass transfer across the boundary, but energy transfer exists.
Explanation:
- A closed thermodynamic system is a system in which there is no mass transfer across the boundary. This means that the total mass of the system remains constant.
- However, energy transfer is allowed across the boundary. This means that the system can exchange heat and work with its surroundings.
- The boundary of a closed system may be rigid or flexible, but it does not allow the transfer of mass.
- Examples of closed thermodynamic systems include a piston-cylinder assembly, a water-filled radiator, or a sealed container of gas.
- In a closed system, the energy transfer can occur in the form of heat or work.
- Heat transfer can occur through conduction, convection, or radiation, depending on the specific system.
- Work transfer can occur due to mechanical work done on or by the system, such as compression or expansion of a gas.
- The energy transfer in a closed system obeys the first law of thermodynamics, which states that the change in internal energy of a system is equal to the heat added to the system minus the work done by the system.
Summary:
- A closed thermodynamic system is one in which there is no mass transfer across the boundary, but energy transfer exists.
- The system can exchange heat and work with its surroundings, but the total mass of the system remains constant.
Note: Remember to always double-check the answer and the explanation provided.
Explanation:
- A closed thermodynamic system is a system in which there is no mass transfer across the boundary. This means that the total mass of the system remains constant.
- However, energy transfer is allowed across the boundary. This means that the system can exchange heat and work with its surroundings.
- The boundary of a closed system may be rigid or flexible, but it does not allow the transfer of mass.
- Examples of closed thermodynamic systems include a piston-cylinder assembly, a water-filled radiator, or a sealed container of gas.
- In a closed system, the energy transfer can occur in the form of heat or work.
- Heat transfer can occur through conduction, convection, or radiation, depending on the specific system.
- Work transfer can occur due to mechanical work done on or by the system, such as compression or expansion of a gas.
- The energy transfer in a closed system obeys the first law of thermodynamics, which states that the change in internal energy of a system is equal to the heat added to the system minus the work done by the system.
Summary:
- A closed thermodynamic system is one in which there is no mass transfer across the boundary, but energy transfer exists.
- The system can exchange heat and work with its surroundings, but the total mass of the system remains constant.
Note: Remember to always double-check the answer and the explanation provided.
Two blocks of iron and copper, both initially at 800C, are dropped into a large lake at 150C. The mass of the iron and copper blocks are 50kg and 20 kg respectively. After a while, the system is in thermal equilibrium due to heat transfer between blocks and lake water.Total entropy change for this process is:-
Ciron = 0.45 kJ/kg0C, Ccopper = 0.386 kJ/kg0C
- a)-4.579 kJ/K
- b)-1.571kJ/K
- c)0.670 kJ/K
- d)6.820 kJ/K
Correct answer is option 'C'. Can you explain this answer?
Two blocks of iron and copper, both initially at 800C, are dropped into a large lake at 150C. The mass of the iron and copper blocks are 50kg and 20 kg respectively. After a while, the system is in thermal equilibrium due to heat transfer between blocks and lake water.
Total entropy change for this process is:-
Ciron = 0.45 kJ/kg0C, Ccopper = 0.386 kJ/kg0C
Ciron = 0.45 kJ/kg0C, Ccopper = 0.386 kJ/kg0C
a)
-4.579 kJ/K
b)
-1.571kJ/K
c)
0.670 kJ/K
d)
6.820 kJ/K
|
|
Garima Mehta answered |
Answer is (c)
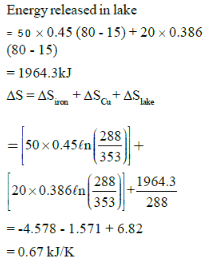
Which of the following aspect is not true regarding microscopic propertie s of thermodynamic system- a)a knowledge of the structure of matter is essential
- b)a limited number of variables/properties are needed to describe the state of matter
- c)the values of these variables cannot be measured
- d)statistical averaging is adopted to predict the behaviour of individual fluid particle
Correct answer is option 'B'. Can you explain this answer?
Which of the following aspect is not true regarding microscopic propertie s of thermodynamic system
a)
a knowledge of the structure of matter is essential
b)
a limited number of variables/properties are needed to describe the state of matter
c)
the values of these variables cannot be measured
d)
statistical averaging is adopted to predict the behaviour of individual fluid particle
|
|
Aditya Chavan answered |
Explanation:
Microscopic properties of a thermodynamic system refer to the properties of individual particles that make up the system. These properties cannot be measured directly but can be inferred through statistical analysis. The following aspects are true regarding microscopic properties of a thermodynamic system:
A. Knowledge of the structure of matter is essential: To understand the microscopic properties of a thermodynamic system, one needs to have knowledge about the structure of matter. This includes information about the behavior of atoms, molecules, and ions that make up the system.
C. The values of these variables cannot be measured: The values of microscopic properties cannot be measured directly. This is because individual particles are too small to be observed directly. However, statistical analysis can be used to infer these properties.
D. Statistical averaging is adopted to predict the behavior of individual fluid particles: Statistical averaging is used to predict the behavior of individual particles. This involves taking an average of the properties of a large number of particles to infer the properties of individual particles.
B. A limited number of variables/properties are needed to describe the state of matter: This aspect is not true. A large number of variables/properties are needed to describe the state of matter at the microscopic level. These include velocity, position, energy, and momentum of individual particles.
Conclusion:
In conclusion, the statement that a limited number of variables/properties are needed to describe the state of matter is not true regarding microscopic properties of a thermodynamic system. Instead, a large number of variables/properties are needed to describe the behavior of individual particles.
Microscopic properties of a thermodynamic system refer to the properties of individual particles that make up the system. These properties cannot be measured directly but can be inferred through statistical analysis. The following aspects are true regarding microscopic properties of a thermodynamic system:
A. Knowledge of the structure of matter is essential: To understand the microscopic properties of a thermodynamic system, one needs to have knowledge about the structure of matter. This includes information about the behavior of atoms, molecules, and ions that make up the system.
C. The values of these variables cannot be measured: The values of microscopic properties cannot be measured directly. This is because individual particles are too small to be observed directly. However, statistical analysis can be used to infer these properties.
D. Statistical averaging is adopted to predict the behavior of individual fluid particles: Statistical averaging is used to predict the behavior of individual particles. This involves taking an average of the properties of a large number of particles to infer the properties of individual particles.
B. A limited number of variables/properties are needed to describe the state of matter: This aspect is not true. A large number of variables/properties are needed to describe the state of matter at the microscopic level. These include velocity, position, energy, and momentum of individual particles.
Conclusion:
In conclusion, the statement that a limited number of variables/properties are needed to describe the state of matter is not true regarding microscopic properties of a thermodynamic system. Instead, a large number of variables/properties are needed to describe the behavior of individual particles.
Zeroth law of thermodynamics form the basis of measurement of- a)pressure
- b)temperature
- c)heat exchanger
- d)work
Correct answer is option 'B'. Can you explain this answer?
Zeroth law of thermodynamics form the basis of measurement of
a)
pressure
b)
temperature
c)
heat exchanger
d)
work
|
|
Shreya Choudhury answered |
Understanding the Zeroth Law of Thermodynamics
The Zeroth Law of Thermodynamics is fundamental in establishing a basis for temperature measurement. Here’s how it works:
Definition of the Zeroth Law
- The Zeroth Law states that if two systems are each in thermal equilibrium with a third system, then they are in thermal equilibrium with each other.
Implications for Temperature Measurement
- This law allows us to define temperature in a way that is consistent and measurable.
- If System A is at the same temperature as System C, and System B is also at the same temperature as System C, then System A and System B must be at the same temperature as well.
Establishing a Temperature Scale
- The Zeroth Law enables the creation of a temperature scale.
- By using a thermometer (System C) that is in thermal equilibrium with another system, we can assign a temperature value to that system.
Practical Applications
- Thermometers, which are based on the principles of the Zeroth Law, allow for the accurate measurement of temperature in various applications.
- This principle is crucial in scientific experiments, industrial processes, and everyday life for ensuring accurate temperature readings.
Conclusion
- Since temperature is a scalar quantity that can be consistently measured using the Zeroth Law, it forms the basis for all temperature measurement techniques, making option 'B' the correct answer.
Understanding the Zeroth Law is essential for anyone studying thermodynamics and its applications in mechanical engineering.
The Zeroth Law of Thermodynamics is fundamental in establishing a basis for temperature measurement. Here’s how it works:
Definition of the Zeroth Law
- The Zeroth Law states that if two systems are each in thermal equilibrium with a third system, then they are in thermal equilibrium with each other.
Implications for Temperature Measurement
- This law allows us to define temperature in a way that is consistent and measurable.
- If System A is at the same temperature as System C, and System B is also at the same temperature as System C, then System A and System B must be at the same temperature as well.
Establishing a Temperature Scale
- The Zeroth Law enables the creation of a temperature scale.
- By using a thermometer (System C) that is in thermal equilibrium with another system, we can assign a temperature value to that system.
Practical Applications
- Thermometers, which are based on the principles of the Zeroth Law, allow for the accurate measurement of temperature in various applications.
- This principle is crucial in scientific experiments, industrial processes, and everyday life for ensuring accurate temperature readings.
Conclusion
- Since temperature is a scalar quantity that can be consistently measured using the Zeroth Law, it forms the basis for all temperature measurement techniques, making option 'B' the correct answer.
Understanding the Zeroth Law is essential for anyone studying thermodynamics and its applications in mechanical engineering.
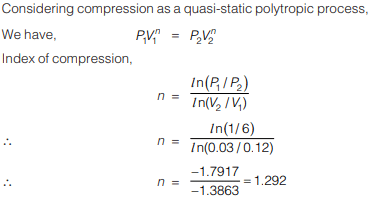
A rigid insulated tank has two equal parts by partition. First part initially contains 5k mol of an ideal gas at
500 kPa, 400C and other part is evacuated. If partition is removed, then the gas fills the entire tank, what will be the total entropy change during this process.- a)28.81 kJ/K
- b)14.4 kJ/K
- c)7.2 kJ/K
- d)21.6 kJ/K
Correct answer is option 'A'. Can you explain this answer?
A rigid insulated tank has two equal parts by partition. First part initially contains 5k mol of an ideal gas at
500 kPa, 400C and other part is evacuated. If partition is removed, then the gas fills the entire tank, what will be the total entropy change during this process.
500 kPa, 400C and other part is evacuated. If partition is removed, then the gas fills the entire tank, what will be the total entropy change during this process.
a)
28.81 kJ/K
b)
14.4 kJ/K
c)
7.2 kJ/K
d)
21.6 kJ/K

|
Anshu Kumar answered |
Answer is (a)
An adiabatic boundary is one which- a)prevents heat transfer
- b)permits heat transfer
- c)prevents work transfer
- d)permits work transfer
Correct answer is option 'A'. Can you explain this answer?
An adiabatic boundary is one which
a)
prevents heat transfer
b)
permits heat transfer
c)
prevents work transfer
d)
permits work transfer

|
Pranab Chaudhary answered |
Adiabatic boundary in thermodynamics refers to a boundary that does not allow any heat transfer between the system and the surroundings. The term adiabatic comes from the Greek word "adiabatos," which means "not to be crossed." This boundary is often used to study and analyze the thermodynamic behavior of different systems.
Heat transfer and adiabatic boundary:
An adiabatic boundary is designed to prevent heat transfer from occurring between the system and the surroundings. This means that the temperature of the system remains constant during any process without any heat transfer. In other words, no heat is lost or gained by the system during any process.
Work transfer and adiabatic boundary:
While an adiabatic boundary prevents heat transfer, it does not prevent work transfer between the system and the surroundings. Work transfer can occur through mechanical means such as compression and expansion of gases. The adiabatic boundary ensures that all the work transfer is converted into internal energy and not lost as heat.
Applications of adiabatic boundary:
Adiabatic boundaries are used in many applications such as:
1. Thermodynamics: Adiabatic boundaries are used to study the thermodynamic behavior of different systems. The behavior of systems can be analyzed by observing the changes in temperature, pressure, and volume during a process.
2. Engineering: Adiabatic boundaries are used in designing and analyzing engines, compressors, and turbines. These devices operate on the principles of thermodynamics, and adiabatic boundaries help in ensuring that the heat losses are minimized.
Conclusion:
In conclusion, an adiabatic boundary is a boundary that does not allow any heat transfer between the system and the surroundings. It is an essential concept in thermodynamics and is used in many applications. Adiabatic boundaries help in studying the thermodynamic behavior of different systems and are used in designing and analyzing various mechanical devices.
Heat transfer and adiabatic boundary:
An adiabatic boundary is designed to prevent heat transfer from occurring between the system and the surroundings. This means that the temperature of the system remains constant during any process without any heat transfer. In other words, no heat is lost or gained by the system during any process.
Work transfer and adiabatic boundary:
While an adiabatic boundary prevents heat transfer, it does not prevent work transfer between the system and the surroundings. Work transfer can occur through mechanical means such as compression and expansion of gases. The adiabatic boundary ensures that all the work transfer is converted into internal energy and not lost as heat.
Applications of adiabatic boundary:
Adiabatic boundaries are used in many applications such as:
1. Thermodynamics: Adiabatic boundaries are used to study the thermodynamic behavior of different systems. The behavior of systems can be analyzed by observing the changes in temperature, pressure, and volume during a process.
2. Engineering: Adiabatic boundaries are used in designing and analyzing engines, compressors, and turbines. These devices operate on the principles of thermodynamics, and adiabatic boundaries help in ensuring that the heat losses are minimized.
Conclusion:
In conclusion, an adiabatic boundary is a boundary that does not allow any heat transfer between the system and the surroundings. It is an essential concept in thermodynamics and is used in many applications. Adiabatic boundaries help in studying the thermodynamic behavior of different systems and are used in designing and analyzing various mechanical devices.
Consider the following statements:1. Thermodynamic properties are the macroscopic coordinates significant only for systems existing in states of thermodynamic equilibrium.
2. Engineering thermodynamic studies about transfer and transformation of energy.
3. Engineering thermodynamics studies about storage, transfer and transformation of energy.Which of the above is/are correct?- a)3 only
- b)1 and 3
- c)2 only
- d)1 and 2
Correct answer is option 'B'. Can you explain this answer?
Consider the following statements:
1. Thermodynamic properties are the macroscopic coordinates significant only for systems existing in states of thermodynamic equilibrium.
2. Engineering thermodynamic studies about transfer and transformation of energy.
3. Engineering thermodynamics studies about storage, transfer and transformation of energy.
2. Engineering thermodynamic studies about transfer and transformation of energy.
3. Engineering thermodynamics studies about storage, transfer and transformation of energy.
Which of the above is/are correct?
a)
3 only
b)
1 and 3
c)
2 only
d)
1 and 2
|
|
Avantika Sen answered |
Explanation:
Thermodynamics is a branch of physics that deals with the study of energy and its transformation, particularly in relation to macroscopic systems. Engineering thermodynamics is an application of thermodynamics to engineering systems and is concerned with the storage, transfer, and transformation of energy.
Statement 1: Thermodynamic properties are the macroscopic coordinates significant only for systems existing in states of thermodynamic equilibrium.
This statement is partially correct. Thermodynamic properties are macroscopic properties that describe the state of a system, such as temperature, pressure, and volume. These properties are significant only for systems existing in states of thermodynamic equilibrium. In a state of thermodynamic equilibrium, the system is in a stable state, with no net change in any of its properties over time.
Statement 2: Engineering thermodynamic studies about transfer and transformation of energy.
This statement is correct. Engineering thermodynamics studies the transfer and transformation of energy in engineering systems. This includes the analysis of heat transfer, work, and energy conversion processes.
Statement 3: Engineering thermodynamics studies about storage, transfer and transformation of energy.
This statement is also correct. Engineering thermodynamics is concerned with the storage, transfer, and transformation of energy. Energy storage can include the use of batteries, capacitors, and other energy storage devices. Energy transfer can include the use of pipes, ducts, and other conduits for the transfer of energy between different parts of a system. Energy transformation can include the conversion of energy from one form to another, such as the conversion of heat energy into mechanical energy in a steam turbine.
Therefore, the correct answer is option 'B' as both statement 1 and statement 3 are correct.
Thermodynamics is a branch of physics that deals with the study of energy and its transformation, particularly in relation to macroscopic systems. Engineering thermodynamics is an application of thermodynamics to engineering systems and is concerned with the storage, transfer, and transformation of energy.
Statement 1: Thermodynamic properties are the macroscopic coordinates significant only for systems existing in states of thermodynamic equilibrium.
This statement is partially correct. Thermodynamic properties are macroscopic properties that describe the state of a system, such as temperature, pressure, and volume. These properties are significant only for systems existing in states of thermodynamic equilibrium. In a state of thermodynamic equilibrium, the system is in a stable state, with no net change in any of its properties over time.
Statement 2: Engineering thermodynamic studies about transfer and transformation of energy.
This statement is correct. Engineering thermodynamics studies the transfer and transformation of energy in engineering systems. This includes the analysis of heat transfer, work, and energy conversion processes.
Statement 3: Engineering thermodynamics studies about storage, transfer and transformation of energy.
This statement is also correct. Engineering thermodynamics is concerned with the storage, transfer, and transformation of energy. Energy storage can include the use of batteries, capacitors, and other energy storage devices. Energy transfer can include the use of pipes, ducts, and other conduits for the transfer of energy between different parts of a system. Energy transformation can include the conversion of energy from one form to another, such as the conversion of heat energy into mechanical energy in a steam turbine.
Therefore, the correct answer is option 'B' as both statement 1 and statement 3 are correct.
A refrigerator transfers 1kJ of heat from a cold region at -200C to hot region at 300C. If COP of refrigerator is 4, total entropy change of region’s will be:-- a)1.72 X 10-4 KJ/K
- b)0.173 KJ/K
- c)1.7 X 10-2 KJ/K
- d)0.9263 KJ/K
Correct answer is option 'A'. Can you explain this answer?
A refrigerator transfers 1kJ of heat from a cold region at -200C to hot region at 300C. If COP of refrigerator is 4, total entropy change of region’s will be:-
a)
1.72 X 10-4 KJ/K
b)
0.173 KJ/K
c)
1.7 X 10-2 KJ/K
d)
0.9263 KJ/K

|
Sanchita Pillai answered |
Answer is (a)
Which of the following is used for measuring high temperature beyond 1063°C?- a)Platinum-platinum/Rhodium thermocouple
- b)Electrical resistance thermometer
- c)Optical method using planck’s law of thermal radiation
- d)Constant pressure gas thermometer
Correct answer is option 'C'. Can you explain this answer?
Which of the following is used for measuring high temperature beyond 1063°C?
a)
Platinum-platinum/Rhodium thermocouple
b)
Electrical resistance thermometer
c)
Optical method using planck’s law of thermal radiation
d)
Constant pressure gas thermometer

|
Abhay Banerjee answered |
0 - 660°C → Platinum resistance thermocouple -190 to 0°C → Platinum-platinum/Rhodium Thermocouple > 1063°C → Planck’s law of thermal radiation.
Which one of the following represents open thermodynamic system?- a)Manual ice cream freezer
- b)Centrifugal pump
- c)Pressure cooker
- d)Bomb calorimeter
Correct answer is option 'B'. Can you explain this answer?
Which one of the following represents open thermodynamic system?
a)
Manual ice cream freezer
b)
Centrifugal pump
c)
Pressure cooker
d)
Bomb calorimeter
|
|
Nayanika Yadav answered |
Open Thermodynamic System:
An open thermodynamic system is one that can exchange both energy and matter with its surroundings. In other words, it allows the transfer of both heat and mass across its boundaries. This type of system is commonly found in many industrial processes and natural phenomena.
Different Options:
Let's analyze each option to determine which one represents an open thermodynamic system:
a) Manual ice cream freezer: This option does not represent an open thermodynamic system. It is a closed system where heat is transferred from the surroundings to the ice cream mixture to freeze it. However, there is no exchange of matter with the surroundings.
b) Centrifugal pump: This option represents an open thermodynamic system. A centrifugal pump operates by continuously drawing in fluid from its surroundings (suction side) and discharging it (discharge side). This process involves the transfer of both energy (work done on the fluid) and matter (fluid flow) across the boundaries of the pump.
c) Pressure cooker: This option does not represent an open thermodynamic system. A pressure cooker is a closed system where the pressure inside increases due to the trapped steam. While there is an exchange of energy (heat transfer) with the surroundings, there is no exchange of matter.
d) Bomb calorimeter: This option does not represent an open thermodynamic system. A bomb calorimeter is a closed system used to measure the heat of combustion of a substance. It does not allow the exchange of matter with the surroundings.
Conclusion:
From the given options, the centrifugal pump (option B) represents an open thermodynamic system because it allows the transfer of both energy and matter across its boundaries. The other options either do not allow the exchange of matter (options a and d) or are completely closed systems (option c).
An open thermodynamic system is one that can exchange both energy and matter with its surroundings. In other words, it allows the transfer of both heat and mass across its boundaries. This type of system is commonly found in many industrial processes and natural phenomena.
Different Options:
Let's analyze each option to determine which one represents an open thermodynamic system:
a) Manual ice cream freezer: This option does not represent an open thermodynamic system. It is a closed system where heat is transferred from the surroundings to the ice cream mixture to freeze it. However, there is no exchange of matter with the surroundings.
b) Centrifugal pump: This option represents an open thermodynamic system. A centrifugal pump operates by continuously drawing in fluid from its surroundings (suction side) and discharging it (discharge side). This process involves the transfer of both energy (work done on the fluid) and matter (fluid flow) across the boundaries of the pump.
c) Pressure cooker: This option does not represent an open thermodynamic system. A pressure cooker is a closed system where the pressure inside increases due to the trapped steam. While there is an exchange of energy (heat transfer) with the surroundings, there is no exchange of matter.
d) Bomb calorimeter: This option does not represent an open thermodynamic system. A bomb calorimeter is a closed system used to measure the heat of combustion of a substance. It does not allow the exchange of matter with the surroundings.
Conclusion:
From the given options, the centrifugal pump (option B) represents an open thermodynamic system because it allows the transfer of both energy and matter across its boundaries. The other options either do not allow the exchange of matter (options a and d) or are completely closed systems (option c).
Comprehension
A football was inflated to a gauge pressure of 1 bar when the ambient temperature was 15°C. When the game started next day, the air temperature at the stadium was 5°C. Assume that the volume of the football remains constant at 2500 cm3.
Gauge pressure of air to which the ball must have been originally inflated so that it would equal 1 bar gauge at the stadium is- a)2.23 bar
- b)1.94 bar
- c)1.07 bar
- d)1.00 bar
Correct answer is option 'C'. Can you explain this answer?
Comprehension
A football was inflated to a gauge pressure of 1 bar when the ambient temperature was 15°C. When the game started next day, the air temperature at the stadium was 5°C. Assume that the volume of the football remains constant at 2500 cm3.
Gauge pressure of air to which the ball must have been originally inflated so that it would equal 1 bar gauge at the stadium is
A football was inflated to a gauge pressure of 1 bar when the ambient temperature was 15°C. When the game started next day, the air temperature at the stadium was 5°C. Assume that the volume of the football remains constant at 2500 cm3.
Gauge pressure of air to which the ball must have been originally inflated so that it would equal 1 bar gauge at the stadium is
a)
2.23 bar
b)
1.94 bar
c)
1.07 bar
d)
1.00 bar

|
Pk Academy answered |
Step-by-Step Solution
- Understand the Relationship Between Pressure and Temperature:
- For a gas at constant volume, the pressure and temperature are related by the formula:
P₁ / T₁ = P₂ / T₂ - Here, P represents absolute pressure and T represents temperature in Kelvin.
- For a gas at constant volume, the pressure and temperature are related by the formula:
- Convert Temperatures to Kelvin:
- T₁ (Initial Temperature) = 15°C + 273 = 288 K
- T₂ (Final Temperature) = 5°C + 273 = 278 K
- Determine Absolute Pressures:
- Gauge Pressure is the pressure above atmospheric pressure.
- Assume atmospheric pressure P_atm = 1 bar.
- Final Absolute Pressure P₂_abs = Gauge Pressure + Atmospheric Pressure = 1 bar + 1 bar = 2 bar
- Apply the Pressure-Temperature Relationship:
- Using the formula: P₁_abs / T₁ = P₂_abs / T₂
- Substitute the known values:
P₁_abs = (P₂_abs × T₁) / T₂ = (2 bar × 288 K) / 278 K ≈ 2.07 bar
- Calculate the Original Gauge Pressure:
- Gauge Pressure P₁_gauge = Absolute Pressure - Atmospheric Pressure
- P₁_gauge = 2.07 bar - 1 bar = 1.07 bar
Conclusion
The original gauge pressure to which the football must have been inflated is 1.07 bar. Therefore, the correct answer is:
Option c) 1.07 bar
Air undergoes a polytropic process in an adiabatic nozzle with n = 1.2. Inlet state of air is 800 kPa, 1230C with a velocity of 32 m/s and exit state is 300 kPa. Find out the velocity of air at nozzle exit.- a)654 m/s
- b)348 m/s
- c)244 m/s
- d)436 m/s
Correct answer is option 'B'. Can you explain this answer?
Air undergoes a polytropic process in an adiabatic nozzle with n = 1.2. Inlet state of air is 800 kPa, 1230C with a velocity of 32 m/s and exit state is 300 kPa. Find out the velocity of air at nozzle exit.
a)
654 m/s
b)
348 m/s
c)
244 m/s
d)
436 m/s
|
|
Debolina Menon answered |
To find the velocity of air at the nozzle exit, we can use the equation for a polytropic process:
P1 * V1^n = P2 * V2^n
Where:
P1 = Inlet pressure
V1 = Inlet velocity
n = Polytropic index
P2 = Exit pressure
V2 = Exit velocity
Given data:
P1 = 800 kPa
V1 = 32 m/s
n = 1.2
P2 = 300 kPa
Let's solve for V2:
800 * (32)^1.2 = 300 * V2^1.2
First, let's calculate (32)^1.2:
(32)^1.2 = 32 * (32)^0.2 ≈ 32 * 1.58489 ≈ 50.717
Now, let's rearrange the equation to solve for V2:
V2^1.2 = (800 * 50.717) / 300
V2^1.2 ≈ 134.96
Taking the 1.2th root of both sides:
V2 ≈ 134.96^(1/1.2)
V2 ≈ 48.51 m/s
Therefore, the velocity of air at the nozzle exit is approximately 48.51 m/s.
Since none of the given options match the calculated velocity, it seems there may be an error in the question or answer choices.
P1 * V1^n = P2 * V2^n
Where:
P1 = Inlet pressure
V1 = Inlet velocity
n = Polytropic index
P2 = Exit pressure
V2 = Exit velocity
Given data:
P1 = 800 kPa
V1 = 32 m/s
n = 1.2
P2 = 300 kPa
Let's solve for V2:
800 * (32)^1.2 = 300 * V2^1.2
First, let's calculate (32)^1.2:
(32)^1.2 = 32 * (32)^0.2 ≈ 32 * 1.58489 ≈ 50.717
Now, let's rearrange the equation to solve for V2:
V2^1.2 = (800 * 50.717) / 300
V2^1.2 ≈ 134.96
Taking the 1.2th root of both sides:
V2 ≈ 134.96^(1/1.2)
V2 ≈ 48.51 m/s
Therefore, the velocity of air at the nozzle exit is approximately 48.51 m/s.
Since none of the given options match the calculated velocity, it seems there may be an error in the question or answer choices.
Common data for 6, 7 & 8:
In adiabatic turbine, air enters at 550 kPa, 425K and leaves at 110kPa, 325K. The inlet and exit velocities of air are 150m/s and 50m/s respectively
T0 = 250C, CP = 1.005 kJ/kg-K, R = 0.287 kJ/kg-K
Q. Isentropic efficiency is: -
- a)47%
- b)52.6%
- c)29%
- d)66.2%
Correct answer is option 'D'. Can you explain this answer?
Common data for 6, 7 & 8:
In adiabatic turbine, air enters at 550 kPa, 425K and leaves at 110kPa, 325K. The inlet and exit velocities of air are 150m/s and 50m/s respectively
In adiabatic turbine, air enters at 550 kPa, 425K and leaves at 110kPa, 325K. The inlet and exit velocities of air are 150m/s and 50m/s respectively
T0 = 250C, CP = 1.005 kJ/kg-K, R = 0.287 kJ/kg-K
Q. Isentropic efficiency is: -
a)
47%
b)
52.6%
c)
29%
d)
66.2%

|
Arya Kaur answered |

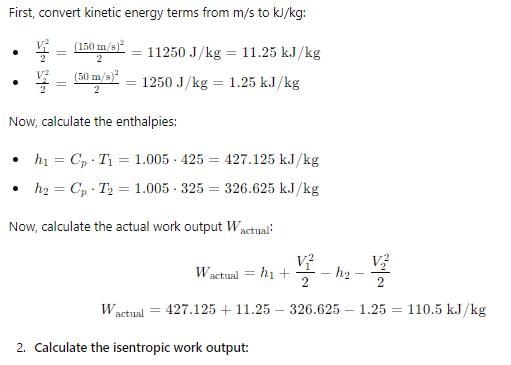
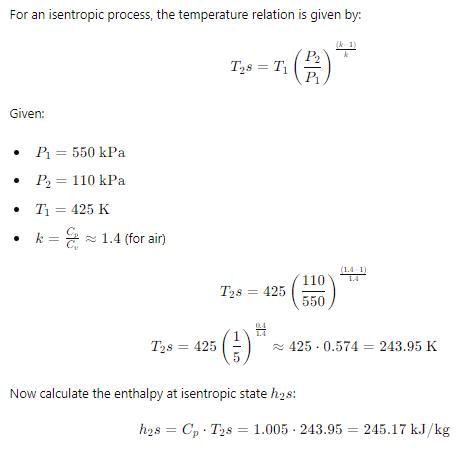

Athermodynamic system refers to- a)any defined region in space
- b)a specified mass in fluid flow
- c)a specified region of constant volume
- d)a prescribed and identifiable quantity of mat
Correct answer is option 'D'. Can you explain this answer?
Athermodynamic system refers to
a)
any defined region in space
b)
a specified mass in fluid flow
c)
a specified region of constant volume
d)
a prescribed and identifiable quantity of mat

|
Aaditya Jain answered |
A certain quantity of matter or a region in space upon which attention is focused in the analysis of a problem is called a system.
Chapter doubts & questions for Basic Concepts & Zeroth Law of Thermodynamics - Mechanical Engineering Optional Notes for UPSC 2025 is part of UPSC CSE exam preparation. The chapters have been prepared according to the UPSC CSE exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for UPSC CSE 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Basic Concepts & Zeroth Law of Thermodynamics - Mechanical Engineering Optional Notes for UPSC in English & Hindi are available as part of UPSC CSE exam.
Download more important topics, notes, lectures and mock test series for UPSC CSE Exam by signing up for free.
Mechanical Engineering Optional Notes for UPSC
316 videos|511 docs|265 tests
|
Related UPSC CSE Content

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up within 7 days!
Access 1000+ FREE Docs, Videos and Tests
Takes less than 10 seconds to signup