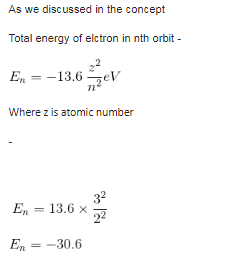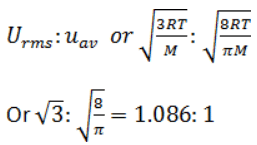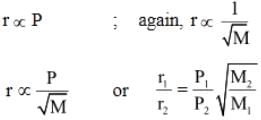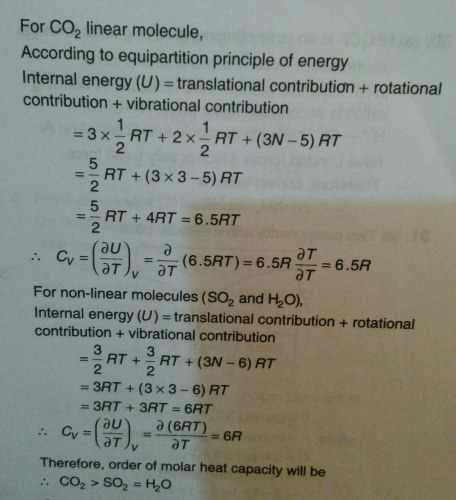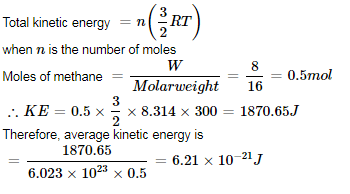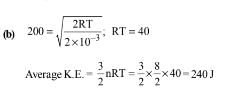All Exams >
Chemistry >
Physical Chemistry >
All Questions
All questions of Theory of Gases for Chemistry Exam
Maximum number of electrons in a subshell with l = 3 and n = 4 is- a)10
- b)12
- c)14
- d)16
Correct answer is option 'C'. Can you explain this answer?
Maximum number of electrons in a subshell with l = 3 and n = 4 is
a)
10
b)
12
c)
14
d)
16
|
|
Chirag Verma answered |
n = 4, l =3, which denotes 4f subshell.
In f subshell, there are 7 orbitals and each orbital can accommodate a maximum of two electrons, so, maximum no. of electrons in 4f subshell = 7 × 2 = 14
In f subshell, there are 7 orbitals and each orbital can accommodate a maximum of two electrons, so, maximum no. of electrons in 4f subshell = 7 × 2 = 14
Formula to find out the no. of electrons = 4l+ 2
∵ l= 3
⇒ 4 × 3 + 2 = 14
∵ l= 3
⇒ 4 × 3 + 2 = 14
Thus, there are 14 electrons.
Among the following curves, which is not according to Charle’s law:- a)
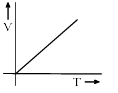
- b)
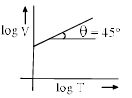
- c)
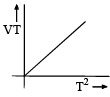
- d)
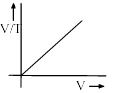
Correct answer is 'D'. Can you explain this answer?
Among the following curves, which is not according to Charle’s law:
a)
b)
c)
d)
|
|
Pooja Choudhury answered |
According to Charles Law, V ∝ T.
i.e. V=kT, where k is proportionality constant.
i.e. V=kT, where k is proportionality constant.
For A: Charles law is in the format of y=mx
Therefore it is a correct representation.
For B: on taking log on both sides in Charles law we get, log V= log T + log k
i.e. y = mx + c, where m = 1 = tan 45º
Therefore it is a correct representation.
For C: on multiplying with T on both sides we get, VT ∝ T2
Therefore it is a correct representation.
For D: ∵ V/T = constant
Therefore it is an incorrect representation.
Therefore it is a correct representation.
For B: on taking log on both sides in Charles law we get, log V= log T + log k
i.e. y = mx + c, where m = 1 = tan 45º
Therefore it is a correct representation.
For C: on multiplying with T on both sides we get, VT ∝ T2
Therefore it is a correct representation.
For D: ∵ V/T = constant
Therefore it is an incorrect representation.
The compressibility factor for an ideal gas is:- a)1.5
- b)1.0
- c)2.0
- d)∞
Correct answer is option 'B'. Can you explain this answer?
The compressibility factor for an ideal gas is:
a)
1.5
b)
1.0
c)
2.0
d)
∞
|
|
Pooja Choudhury answered |
The deviation of ideal behaviour is introduced by compressibility factor Z.
i.e. Z = PV/nRT
for ideal gas PV = nRT, therefore, Z is 1.
i.e. Z = PV/nRT
for ideal gas PV = nRT, therefore, Z is 1.
The root mean square speed of 8 g of He is 300 ms–1. Total kinetic energy of He gas is:
- a)360 J
- b)240 J
- c)120 J
- d)None of these
Correct answer is option 'A'. Can you explain this answer?
The root mean square speed of 8 g of He is 300 ms–1. Total kinetic energy of He gas is:
a)
360 J
b)
240 J
c)
120 J
d)
None of these

|
Anushka Basak answered |
Calculation of Total Kinetic Energy of He Gas
Root Mean Square Speed of He Gas = 300 m/s
Mass of He Gas (m) = 8 g = 0.008 kg (as 1 g = 0.001 kg)
Boltzmann Constant (k) = 1.38 × 10^-23 J/K (given)
Using the formula for calculating Root Mean Square Speed,
vRMS = √(3kT/m)
where T is the temperature of the gas.
Rearranging the formula, we get
T = m*vRMS^2 / 3k
Substituting the given values, we get
T = (0.008 kg)*(300 m/s)^2 / (3*1.38 × 10^-23 J/K)
T = 2.603 × 10^5 K
Using the formula for calculating Total Kinetic Energy of a Gas,
KE = (3/2)*nRT
where n is the number of moles of gas, R is the gas constant, and T is the temperature of the gas.
Since we know the temperature and mass of He gas, we can calculate the number of moles using the formula
n = m/M
where M is the molar mass of He gas (4 g/mol).
Substituting the values, we get
n = 0.008 kg / 4 g/mol = 0.002 mol
Substituting the values of n, R, and T, we get
KE = (3/2)*(0.002 mol)*(8.314 J/mol·K)*(2.603 × 10^5 K)
KE = 119.9 J ≈ 120 J
Therefore, the total kinetic energy of He gas is 120 J (option A is correct).
Root Mean Square Speed of He Gas = 300 m/s
Mass of He Gas (m) = 8 g = 0.008 kg (as 1 g = 0.001 kg)
Boltzmann Constant (k) = 1.38 × 10^-23 J/K (given)
Using the formula for calculating Root Mean Square Speed,
vRMS = √(3kT/m)
where T is the temperature of the gas.
Rearranging the formula, we get
T = m*vRMS^2 / 3k
Substituting the given values, we get
T = (0.008 kg)*(300 m/s)^2 / (3*1.38 × 10^-23 J/K)
T = 2.603 × 10^5 K
Using the formula for calculating Total Kinetic Energy of a Gas,
KE = (3/2)*nRT
where n is the number of moles of gas, R is the gas constant, and T is the temperature of the gas.
Since we know the temperature and mass of He gas, we can calculate the number of moles using the formula
n = m/M
where M is the molar mass of He gas (4 g/mol).
Substituting the values, we get
n = 0.008 kg / 4 g/mol = 0.002 mol
Substituting the values of n, R, and T, we get
KE = (3/2)*(0.002 mol)*(8.314 J/mol·K)*(2.603 × 10^5 K)
KE = 119.9 J ≈ 120 J
Therefore, the total kinetic energy of He gas is 120 J (option A is correct).
Rate of diffusion of a gas is:- a)Directly proportional to its density
- b)Directly proportional to its molecular weight
- c)Directly proportional to the square root of its molecular weight.
- d)Inversely proportional to the square root of its molecular weight.
Correct answer is option 'D'. Can you explain this answer?
Rate of diffusion of a gas is:
a)
Directly proportional to its density
b)
Directly proportional to its molecular weight
c)
Directly proportional to the square root of its molecular weight.
d)
Inversely proportional to the square root of its molecular weight.
|
|
Neha Choudhury answered |
Graham's law states that the rate of diffusion or of effusion of a gas is inversely proportional to the square root of its molecular weight.
Rate is proportional to 1/ √MW
Thus, if the molecular weight of one gas is four times that of another, it would diffuse through a porous plug or escape through a small pinhole in a vessel at half the rate of the other (heavier gases diffuse more slowly).
The value of van der Waals’ constant ‘a’ for the gases O2, N2, NH3 and CH4 are 1.360, 1.390, 4.170 and 2.253 L2 atm mol–2 respectively. The gas which can most easily be liquefied is:- a)O2
- b)N2
- c)NH3
- d)CH4
Correct answer is option 'C'. Can you explain this answer?
The value of van der Waals’ constant ‘a’ for the gases O2, N2, NH3 and CH4 are 1.360, 1.390, 4.170 and 2.253 L2 atm mol–2 respectively. The gas which can most easily be liquefied is:
a)
O2
b)
N2
c)
NH3
d)
CH4

|
Rahul Chatterjee answered |
The ease of liquification of a gas depends on their intermolecular force of attraction which in turn is measured in terms of van der Waals’ constant ‘a’. Hence, higher the value of ‘a’, greater the intermolecular force of attraction, easier the liquification. In the present case, NH3 has highest ‘a’, can most easily be liquefied.
Helium atom is two times heavier than a hydrogen molecule. At 298 K, the average kinetic energy of a helium atom is:- a)Two times that of a hydrogen molecule
- b)Same as that of a hydrogen molecule
- c)Four times that of a hydrogen mo lecule
- d)Half that of a hydrogen mo lecule
Correct answer is 'B'. Can you explain this answer?
Helium atom is two times heavier than a hydrogen molecule. At 298 K, the average kinetic energy of a helium atom is:
a)
Two times that of a hydrogen molecule
b)
Same as that of a hydrogen molecule
c)
Four times that of a hydrogen mo lecule
d)
Half that of a hydrogen mo lecule

|
Baishali Bajaj answered |
Average kinetic energy depends only on temperature and does not depend upon the nature of the gas.
Equal weights of methane and oxygen and mixed in an empty container at 25°C. The fraction of the total pressure exerted by oxygen is:- a)1/3
- b)½
- c)2/3
- d)(1/3) × (273/298)
Correct answer is 'A'. Can you explain this answer?
Equal weights of methane and oxygen and mixed in an empty container at 25°C. The fraction of the total pressure exerted by oxygen is:
a)
1/3
b)
½
c)
2/3
d)
(1/3) × (273/298)
|
|
Preeti Iyer answered |
Molar mass of methane = 16.042g mol-1 Molar mass of oxygen = 32.00 g mol-1 Therefore if say, 32g of methane and 32g of oxygen mixed,there is 2 moles of methane and 1 moles of oxygen. n= PV/RT pressure is directly proportional to the number of moles Oxygen is 1/3 of total number of moles and hence it exerts 1/3 of the total pressure The answer is a.
The relationship between the van der Waals’ b coefficient of N2 and O2 is:- a)b(N2) = b(O2) = 0
- b)b(N2) = b(O2) ≠ 0
- c)b(N2) > b(O2)
- d)b(N2) < b(O2)
Correct answer is option 'C'. Can you explain this answer?
The relationship between the van der Waals’ b coefficient of N2 and O2 is:
a)
b(N2) = b(O2) = 0
b)
b(N2) = b(O2) ≠ 0
c)
b(N2) > b(O2)
d)
b(N2) < b(O2)
|
|
Saurabh Chavan answered |
B directly proportional to r^3. radius of N2 is 65pm and O2 is 60pm. hence N2>O2.
A gas described by van der Waals’ equation:- a)Behaves similar to an ideal gas in the limit of large volumes
- b)Behaves similar to an ideal gas in the limit of large pressure.
- c)Is characterized by van der Waals’ coefficients that are dependent on the identity of the gas but are independent of the temperature.
- d)Has the pressure that is lower than the pressure exerted by the same gas behaving ideally
Correct answer is option 'A,C'. Can you explain this answer?
A gas described by van der Waals’ equation:
a)
Behaves similar to an ideal gas in the limit of large volumes
b)
Behaves similar to an ideal gas in the limit of large pressure.
c)
Is characterized by van der Waals’ coefficients that are dependent on the identity of the gas but are independent of the temperature.
d)
Has the pressure that is lower than the pressure exerted by the same gas behaving ideally
|
|
Pooja Choudhury answered |
For a real gas
(P + an2/V2)(V – nb) = nRT
- Volume large => a=0 and V-nb = V. Hence it reduces to PV = nRT
- P large but V can’t be neglected. So, P(V-nb) = nRT
- a and b are temperature independent.
- For real gas a is not 0 but ideal gas has a = 0. Hence ideal gas exerts more pressure on the container walls.
From the above discussion, A, B and D are correct.
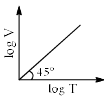
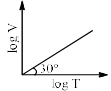
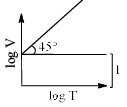
For a closed (not rigid) container containing n = 10 moles of an ideal gas fitted with movable, frictionless, weightless piston operating such that pressure of gas remains constant at 0.821 atm, which graph represents correct variation of log V vs log T where V is in litre and T in Kelvin:- a)
- b)
- c)
- d)
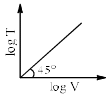
Correct answer is option 'C'. Can you explain this answer?
For a closed (not rigid) container containing n = 10 moles of an ideal gas fitted with movable, frictionless, weightless piston operating such that pressure of gas remains constant at 0.821 atm, which graph represents correct variation of log V vs log T where V is in litre and T in Kelvin:
a)
b)
c)
d)
|
|
Pooja Choudhury answered |
According to Charles Law, V ∝ T.
i.e. V=kT, where k is proportionality constant.
On taking log on both sides in Charles law we get, log V= log T + log k
i.e. y = mx + c, where m = 1 = tan 45º.
On comparing with the Ideal gas equation,
k = V/T = nR/P
substituting values,
k = (10 * 0.0821)/0.821 = 1
i.e. V=kT, where k is proportionality constant.
On taking log on both sides in Charles law we get, log V= log T + log k
i.e. y = mx + c, where m = 1 = tan 45º.
On comparing with the Ideal gas equation,
k = V/T = nR/P
substituting values,
k = (10 * 0.0821)/0.821 = 1
⇒ log V= log T + log 1
i.e. log V= log T
i.e. log V= log T
Which in not correct curve for gay-lussac’s law:- a)
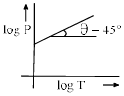
- b)

- c)
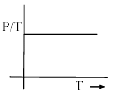
- d)
Correct answer is option 'B'. Can you explain this answer?
Which in not correct curve for gay-lussac’s law:
a)
b)

c)
d)

|
|
Pooja Choudhury answered |
According to Gay-Lussac's Law, P ∝ T.
i.e. P =kT, where k is proportionality constant.
i.e. P =kT, where k is proportionality constant.
For A: On taking log on both sides in Gay-Lussac's Law we get, log P= log T + log k
i.e. y = mx + c, where m = 1 = tan 45º
Therefore it is a correct representation.
For B: ∵ P/T = constant
Therefore it is an incorrect representation.
For C: ∵ P/T = constant
Therefore it is a correct representation.
For D: Gay-Lussac's Law is in the format of y=mx
Therefore it is a correct representation.
i.e. y = mx + c, where m = 1 = tan 45º
Therefore it is a correct representation.
For B: ∵ P/T = constant
Therefore it is an incorrect representation.
For C: ∵ P/T = constant
Therefore it is a correct representation.
For D: Gay-Lussac's Law is in the format of y=mx
Therefore it is a correct representation.
Indicate the correct statement for equal volume of N2(g) and CO2(g) at 25°C and 1 atm:
- a)The average translat ional K.E. per molecule is the same for N2 and CO2
- b)The density of N2 is less than that of CO2
- c)All of these
- d)The total translational K.E. of both N2 and CO2 is the same
Correct answer is option 'C'. Can you explain this answer?
Indicate the correct statement for equal volume of N2(g) and CO2(g) at 25°C and 1 atm:
a)
The average translat ional K.E. per molecule is the same for N2 and CO2
b)
The density of N2 is less than that of CO2
c)
All of these
d)
The total translational K.E. of both N2 and CO2 is the same

|
Palak Singh answered |
Explanation:
The correct statement for equal volume of N2(g) and CO2(g) at 25C and 1 atm is that the density of N2 is less than that of CO2.
Reasoning:
The density of a gas is directly proportional to its molecular weight. The molecular weight of N2 is 28 g/mol, whereas the molecular weight of CO2 is 44 g/mol. Since the molecular weight of CO2 is greater than that of N2, the density of CO2 is greater than that of N2.
The average translational K.E. per molecule is the same for N2 and CO2:
The average translational kinetic energy per molecule is given by the formula:
1/2 mv2 = (3/2) kT
Where m is the mass of the molecule, v is its velocity, k is the Boltzmann constant, and T is the temperature in kelvin. Since the temperature and the mass of the molecules are the same for both N2 and CO2, the average translational kinetic energy per molecule is also the same.
The rms speed remains same for both N2 and CO2:
The root-mean-square (rms) speed of a gas molecule is given by the formula:
vrms = √(3kT/m)
Where m is the mass of the molecule, k is the Boltzmann constant, and T is the temperature in kelvin. Since the temperature and the mass of the molecules are the same for both N2 and CO2, the rms speed of the molecules is also the same.
The total translational K.E. of both N2 and CO2 is the same:
The total translational kinetic energy of a gas is given by the formula:
KE = (3/2) NkT
Where N is the number of molecules, k is the Boltzmann constant, and T is the temperature in kelvin. Since the temperature and the number of molecules are the same for both N2 and CO2, the total translational kinetic energy of both gases is the same.
Conclusion:
Hence, the correct statement for equal volume of N2(g) and CO2(g) at 25C and 1 atm is that the density of N2 is less than that of CO2.
The correct statement for equal volume of N2(g) and CO2(g) at 25C and 1 atm is that the density of N2 is less than that of CO2.
Reasoning:
The density of a gas is directly proportional to its molecular weight. The molecular weight of N2 is 28 g/mol, whereas the molecular weight of CO2 is 44 g/mol. Since the molecular weight of CO2 is greater than that of N2, the density of CO2 is greater than that of N2.
The average translational K.E. per molecule is the same for N2 and CO2:
The average translational kinetic energy per molecule is given by the formula:
1/2 mv2 = (3/2) kT
Where m is the mass of the molecule, v is its velocity, k is the Boltzmann constant, and T is the temperature in kelvin. Since the temperature and the mass of the molecules are the same for both N2 and CO2, the average translational kinetic energy per molecule is also the same.
The rms speed remains same for both N2 and CO2:
The root-mean-square (rms) speed of a gas molecule is given by the formula:
vrms = √(3kT/m)
Where m is the mass of the molecule, k is the Boltzmann constant, and T is the temperature in kelvin. Since the temperature and the mass of the molecules are the same for both N2 and CO2, the rms speed of the molecules is also the same.
The total translational K.E. of both N2 and CO2 is the same:
The total translational kinetic energy of a gas is given by the formula:
KE = (3/2) NkT
Where N is the number of molecules, k is the Boltzmann constant, and T is the temperature in kelvin. Since the temperature and the number of molecules are the same for both N2 and CO2, the total translational kinetic energy of both gases is the same.
Conclusion:
Hence, the correct statement for equal volume of N2(g) and CO2(g) at 25C and 1 atm is that the density of N2 is less than that of CO2.
At constant volume, for a fixed number of moles of a gas the pressure of the gas increases with rise of temperature due to:- a)Increase in average molecular speed
- b)Increase rate of collisions amongst molecules
- c)Increase in molecular attraction
- d)Decrease in mean freed path
Correct answer is option 'A'. Can you explain this answer?
At constant volume, for a fixed number of moles of a gas the pressure of the gas increases with rise of temperature due to:
a)
Increase in average molecular speed
b)
Increase rate of collisions amongst molecules
c)
Increase in molecular attraction
d)
Decrease in mean freed path
|
|
Aditya Deshmukh answered |
Average speed = √(8RT/πM) ie, at constant volume, for a fixed mass, increasing temperature increases average speeds and molecules collide more frequently to the wall of container leading to increase in gas pressure.
The rate of diffusion of methane at a given temperature is twice that of a gas X. The molecular weight of X is:- a)64.0
- b)32.0
- c)4.0
- d)8.0
Correct answer is option 'A'. Can you explain this answer?
The rate of diffusion of methane at a given temperature is twice that of a gas X. The molecular weight of X is:
a)
64.0
b)
32.0
c)
4.0
d)
8.0

|
Kaavya Sengupta answered |
rate is proportional to inverse of sqrt of mass of gas. r1/r2 = sqrt (Mx/Mmethane) 4 = Mx/Mmethane =>Mx = 4*16 = 64 (mass of methane=16)
A bottle of dry ammonia and a bottle of dry hydrogen chloride connected through a long tube are opened simultaneously at both ends the white ammonium chloride ring first formed will be:- a)At the centre of the tube
- b)Near the hydrogen chloride bottle
- c)Near the ammo nia bottle
- d)Throughout the length of the tube.
Correct answer is option 'B'. Can you explain this answer?
A bottle of dry ammonia and a bottle of dry hydrogen chloride connected through a long tube are opened simultaneously at both ends the white ammonium chloride ring first formed will be:
a)
At the centre of the tube
b)
Near the hydrogen chloride bottle
c)
Near the ammo nia bottle
d)
Throughout the length of the tube.

|
Kaavya Sengupta answered |
According to Graham's law of effusion, rate of effusion is inversely proportional to squareroot of molecular mass.
Rate of effusion = √(1/Molar mass)
That means higher the molar mass will effuse slowly and they move shorterdistance only.
NH3 molar mass is 17 and HCl molar mass is 36.5
So HCl effuses slowly and the formation of NH4Cl white rings are
formed near by HCl
Match the Column-I and Column-II:

- a)A-P, B-S, C-R, D-Q
- b)A-R, B-S, C-P, D-Q
- c)A-Q, B-S, C-R, D-P
- d)A-Q, B-S, C-P, D-R
Correct answer is option 'D'. Can you explain this answer?
Match the Column-I and Column-II:

a)
A-P, B-S, C-R, D-Q
b)
A-R, B-S, C-P, D-Q
c)
A-Q, B-S, C-R, D-P
d)
A-Q, B-S, C-P, D-R

|
Veda Institute answered |
Correct Answer :- D
Explanation : A-Q, B-S, C-P, D-R
At a definite temperature (T), the distribution of velocities is given by the curve. The curve that indicates that the velocity corresponding to points A, B and C are: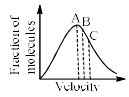
- a)Most probable, root mean square and average
- b)Average, root mean square and most probable
- c)Root mean square, average and most probable
- d)Most probable, average and root mean square
Correct answer is option 'D'. Can you explain this answer?
At a definite temperature (T), the distribution of velocities is given by the curve. The curve that indicates that the velocity corresponding to points A, B and C are:
a)
Most probable, root mean square and average
b)
Average, root mean square and most probable
c)
Root mean square, average and most probable
d)
Most probable, average and root mean square
|
|
Pooja Choudhury answered |
Vrms=√3kT/m
Vavg=√8kT/πm
Vmp=√2kT/m
√3>√8\π>√2 (Vrms >Vavg> Vmp)
Vavg=√8kT/πm
Vmp=√2kT/m
√3>√8\π>√2 (Vrms >Vavg> Vmp)
Speed increases from left to right on the x-axis. Therefore, the root mean square speed is farthest to the right on the graph (means it is largest) and the most probable speed is farthest to the left (means that is small).
At constant volume, for a fixed number of moles of a gas, the pressure of the gas increases with an increase in temperature due to:a) Increase in the average molecule speed b) Increase in rate of collision amongst moleculesc) Increase in molecular attractiond) Increase in mean free pathCorrect answer is option 'A'. Can you explain this answer?

|
Kunal Goyal answered |
Pressure on the walls of the container is equal to the change of momentum per unit time per unit area. At constant volume, for a fixed number of moles of a gas, the pressure increases with rise in temperature due to increase in average molecular speed. This increases the change in momentum during collisions.
Maximum number of electrons in a subshell with l = 3 and n = 4 is- a)10
- b)12
- c)14
- d)16
Correct answer is option 'C'. Can you explain this answer?
Maximum number of electrons in a subshell with l = 3 and n = 4 is
a)
10
b)
12
c)
14
d)
16
|
|
Pooja Choudhury answered |
n = 4, l =3, which denotes 4f subshell.
In f subshell, there are 7 orbitals and each orbital can accommodate a maximum of two electrons, so, maximum no. of electrons in 4f subshell = 7 × 2 = 14
In f subshell, there are 7 orbitals and each orbital can accommodate a maximum of two electrons, so, maximum no. of electrons in 4f subshell = 7 × 2 = 14
Formula to find out the no. of electrons = 4l+ 2
∵ l= 3
⇒ 4 × 3 + 2 = 14
∵ l= 3
⇒ 4 × 3 + 2 = 14
Thus, there are 14 electrons.
The compressibility factor for an ideal gas is:
- a)1.5
- b)2.0
- c)1.0
- d)∞
Correct answer is option 'C'. Can you explain this answer?
The compressibility factor for an ideal gas is:
a)
1.5
b)
2.0
c)
1.0
d)
∞
|
|
Vedika Singh answered |
It may be thought of as the ratio of the actual volume of a real gas to the volume predicted by the ideal gas at the same temperature and pressure as the actual volume. For an ideal gas, Z always has a value of 1.
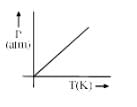
A graph is plotted between P (atm) vs t°C for 10 mol of an ideal gas as follows: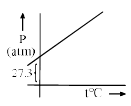 Then slope of curve and volume of container (L) respectively, is
Then slope of curve and volume of container (L) respectively, is- a)0.1, 8.21
- b)8.21, 0.1
- c)27.3, 8.21
- d)8.21, 27.3
Correct answer is option 'A'. Can you explain this answer?
A graph is plotted between P (atm) vs t°C for 10 mol of an ideal gas as follows:
Then slope of curve and volume of container (L) respectively, is
a)
0.1, 8.21
b)
8.21, 0.1
c)
27.3, 8.21
d)
8.21, 27.3
|
|
Pooja Choudhury answered |
According to ideal gas equation,
PV = nRT i.e. P = (nR/V) T
⇒ V = nRT/P = (10 * 0.0821 * 273)/27.3 =8.21
⇒ m = nR/V = (10 * 0.0821)/8.21 = 0.1
PV = nRT i.e. P = (nR/V) T
⇒ V = nRT/P = (10 * 0.0821 * 273)/27.3 =8.21
⇒ m = nR/V = (10 * 0.0821)/8.21 = 0.1
Consider, the two identical containers, one with 1 mole of H2 and the other with 1 mole of He. If the root mean square (RMS) velocities of the two gases are the same, then the ratio of the temperature,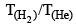 is:
is:- a)1/2
- b)2
- c)
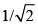
- d)
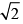
Correct answer is option 'A'. Can you explain this answer?
Consider, the two identical containers, one with 1 mole of H2 and the other with 1 mole of He. If the root mean square (RMS) velocities of the two gases are the same, then the ratio of the temperature,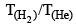 is:
is:
a)
1/2
b)
2
c)
d)
|
|
Pooja Choudhury answered |
V = (3RT/M)1/2
Vrms H2 = (3RTH2/2)1/2
Vrms He = (3RTHe/4)1/2
(3RTH2/2)1/2 = (3RTHe/2)1/2
TH2/THe= 1/2
Vrms H2 = (3RTH2/2)1/2
Vrms He = (3RTHe/4)1/2
(3RTH2/2)1/2 = (3RTHe/2)1/2
TH2/THe= 1/2
Which is the incorrect curve for Boyle’s law?a)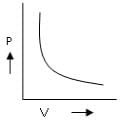 b)
b)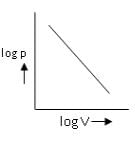 c)
c)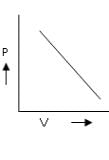 d)
d)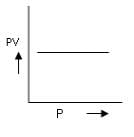 Correct answer is option 'C'. Can you explain this answer?
Correct answer is option 'C'. Can you explain this answer?





|
Edurev.iitjam answered |
According to Boyle's Law, P ∝ 1/V.
For A: Boyle's law is in the format of y=m/x
Therefore it is a correct representation.
For B: On taking log on both sides in Boyle's law we get, log P= - log V + log k
i.e. y = mx + c, where m = -1 = tan 135º
Therefore it is a correct representation.
For C: Boyle's law is in the format of y=m/x
Therefore it is an incorrect representation.
For D: ∵ V/T = constant
Therefore it is a correct representation.
Therefore it is a correct representation.
For B: On taking log on both sides in Boyle's law we get, log P= - log V + log k
i.e. y = mx + c, where m = -1 = tan 135º
Therefore it is a correct representation.
For C: Boyle's law is in the format of y=m/x
Therefore it is an incorrect representation.
For D: ∵ V/T = constant
Therefore it is a correct representation.
The density of neon will be highest at:- a)STP
- b)0°C, 2 atm
- c)273°C, 1 atm
- d)273°C, 2 atm
Correct answer is option 'B'. Can you explain this answer?
The density of neon will be highest at:
a)
STP
b)
0°C, 2 atm
c)
273°C, 1 atm
d)
273°C, 2 atm

|
Anirban Khanna answered |
According to Ideal Gas equation,
PV = nRT
We can also write this equation as:
D = MP/(RT)
where D = density
M = molar mass
P = pressure
R = gas constant
T = temperature
So, density actually depends on P and T if gas is same. D is directly proportional to P and inversely proportional to T.
At STP, P = 1 atm, T = 273 K
2nd option is, P = 2 atm, T = 0 oC = 273 K
3rd option is, T = 273 oC = 546 K
4th option is, P = 2 atm, T = 273 oC = 546 K
In 2nd option, P is high and T is low, so in those conditions density would be highest.
The intermolecular interaction that is dependent on the inverse cube of distance between the molecule is:
a)Ion-ion interactionb)Hydrogen bondc)London forced)Ion-dipole interactionCorrect answer is option 'D'. Can you explain this answer?
|
|
Pooja Choudhury answered |
Ion-ion interaction α Z+ Z-/r2
Ion- dipole interaction α 1/r3
London Dispersion forces α 1/r6
Hydrogen bond force α 1/r
Hence D is correct.
How many degrees of freedom do non linear triatomic gas molecules has?- a)two
- b)six
- c)three
- d)five
Correct answer is option 'B'. Can you explain this answer?
How many degrees of freedom do non linear triatomic gas molecules has?
a)
two
b)
six
c)
three
d)
five
|
|
Hansa Sharma answered |
A triatomic nonlinear gaseous atom has 6 degrees of freedom, that are 3 in all transrational directions and three rotational barriers in all the three axises.
Calculate relative rate of effusion of O2 to CH4 through a container containing O2 and CH4 in 3:2 mass ratio:- a)
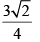
- b)
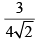
- c)
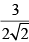
- d)None of these
Correct answer is option 'D'. Can you explain this answer?
Calculate relative rate of effusion of O2 to CH4 through a container containing O2 and CH4 in 3:2 mass ratio:
a)
b)
c)
d)
None of these
|
|
Vikram Kapoor answered |
rO2/rCH4 = nO2/nCH4.(√MCH4/MO2)
= 3/2 × 16/32 × (√16/32)
= 3/(4√2)
= 3/2 × 16/32 × (√16/32)
= 3/(4√2)
No cooling occurs, when an ideal gas undergoes unrestrained expansion, because the molecules a) collide without loss of energy. b) do work equal to loss in kinetic energy. c) are above the inversion temperature. d) exert no attractive force on each other.Correct answer is option 'D'. Can you explain this answer?

|
Mrinalini Sen answered |
According to postulates of kinetic theory, there is no intermolecular attractions or repulsions between the molecules of ideal gases.
Which among the following options do you think has the highest average speed?- a)chlorine
- b)hydrogen
- c)neon
- d)oxygen
Correct answer is option 'B'. Can you explain this answer?
Which among the following options do you think has the highest average speed?
a)
chlorine
b)
hydrogen
c)
neon
d)
oxygen
|
|
Vivek Khatri answered |
The formula of average speed is given by where R is universal gas constant, T is a temperature in Kelvin and M is the mass in kilograms. From the formula, we understand that the average speed is inversely proportional to the root over the mass. As hydrogen has the least mass among the options it has the highest average speed.
where R is universal gas constant, T is a temperature in Kelvin and M is the mass in kilograms. From the formula, we understand that the average speed is inversely proportional to the root over the mass. As hydrogen has the least mass among the options it has the highest average speed.
 where R is universal gas constant, T is a temperature in Kelvin and M is the mass in kilograms. From the formula, we understand that the average speed is inversely proportional to the root over the mass. As hydrogen has the least mass among the options it has the highest average speed.
where R is universal gas constant, T is a temperature in Kelvin and M is the mass in kilograms. From the formula, we understand that the average speed is inversely proportional to the root over the mass. As hydrogen has the least mass among the options it has the highest average speed.A mixture of nitrogen and water vapours is admitted to a flask at 760 torr which contains a sufficient solid drying agent after long time the pressure reached a steady value of 722 torr. If the experiment is done at 27°C and drying agent increases in weight by 0.9 gm, what is the volume of the flask? Neglect any possible vapour of drying agent and volume occupied by drying agent:- a)443.34 L
- b)246.3 L
- c)12.315 L
- d)24.63 L
Correct answer is option 'D'. Can you explain this answer?
A mixture of nitrogen and water vapours is admitted to a flask at 760 torr which contains a sufficient solid drying agent after long time the pressure reached a steady value of 722 torr. If the experiment is done at 27°C and drying agent increases in weight by 0.9 gm, what is the volume of the flask? Neglect any possible vapour of drying agent and volume occupied by drying agent:
a)
443.34 L
b)
246.3 L
c)
12.315 L
d)
24.63 L

|
Kiran Pillai answered |
Ans.


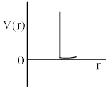
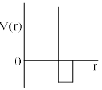
One mole of a monoatomic real gas sat isfied the equation p(V – b) = RT where b is a constant. The relationship of interatomic potential V ( r) and interatomic distance r for the gas is given by:- a)
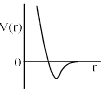
- b)
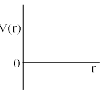
- c)
- d)
Correct answer is option 'C'. Can you explain this answer?
One mole of a monoatomic real gas sat isfied the equation p(V – b) = RT where b is a constant. The relationship of interatomic potential V ( r) and interatomic distance r for the gas is given by:
a)
b)
c)
d)

|
Asf Institute answered |
For a real gas
(P + an2/V2)(V – nb) = nRT
ATQ: P (V-nb) = RT
The extra term “an2/V2” comes because of the attractive forces, hence with a certain decrease in r V(r) remains constant.
But after a huge decrease repulsive forces will dominate and hence V(r) will increase abruptly.
Hence C is correct.
The critical temperature of water is higher than that of O2 because the H2O molecule has:- a)Fewer electrons than O2
- b)Two covalent bonds
- c)V-shape
- d)Dipole moment.
Correct answer is option 'D'. Can you explain this answer?
The critical temperature of water is higher than that of O2 because the H2O molecule has:
a)
Fewer electrons than O2
b)
Two covalent bonds
c)
V-shape
d)
Dipole moment.

|
Anirban Khanna answered |
H2O has bent shape structure that is some acute angle b/w the two hydrogens whereas the oxygen molecule has double bonded with sp2 hy. has 180 degree ange straight woth another O atom.
And therefore, dipole moment is inversely proportionak to the anglle b/w the molecules.
A kid has been playing with an inflated balloon. Suddenly, a reaction happens inside the balloon. This causes the inside surface of the balloon to become sticky, so that some of the air molecules inside which collide against the surface get stuck to it. As a result, immediately after the reaction, the balloon will:- a)Inflate
- b)Deflate
- c)Remain unaltered.
- d)Will change its shape without changing its volume
Correct answer is option 'B'. Can you explain this answer?
A kid has been playing with an inflated balloon. Suddenly, a reaction happens inside the balloon. This causes the inside surface of the balloon to become sticky, so that some of the air molecules inside which collide against the surface get stuck to it. As a result, immediately after the reaction, the balloon will:
a)
Inflate
b)
Deflate
c)
Remain unaltered.
d)
Will change its shape without changing its volume

|
Ramjayakumar Venkatesh answered |
The shape of the balloon in maintained by the constant motion of gas molecules filled inside it. As the gas molecules start sticking to the surface, it's motion decreases and the balloon gets deflated.
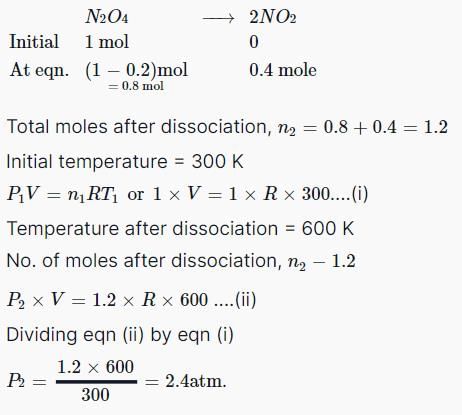
A certain hydrate has the formula MgSO4.xH2O. a quantity of 54.2 g of the compound is heated in an over to drive off the water. If the steam generated exerts a pressure of 24.8 atm in a 2.0 L container at 120°C, calculate x:
- a)2
- b)5
- c)7
- d)6
Correct answer is option 'C'. Can you explain this answer?
A certain hydrate has the formula MgSO4.xH2O. a quantity of 54.2 g of the compound is heated in an over to drive off the water. If the steam generated exerts a pressure of 24.8 atm in a 2.0 L container at 120°C, calculate x:
a)
2
b)
5
c)
7
d)
6

|
Mihir Singh answered |
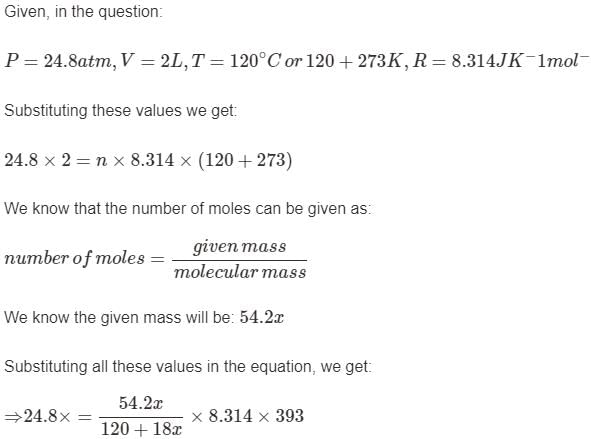
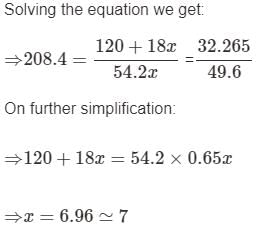
The average velocity of anideal gas molecule at 27°C is 0.3 m/s. The average velocity at 927°C will be:- a)0.6 m/s
- b)0.3 m/s
- c)0.9 m/s
- d)3.0 m/s
Correct answer is option 'D'. Can you explain this answer?
The average velocity of anideal gas molecule at 27°C is 0.3 m/s. The average velocity at 927°C will be:
a)
0.6 m/s
b)
0.3 m/s
c)
0.9 m/s
d)
3.0 m/s
|
|
Chirag Verma answered |
We know,
V1/V2 = [T1/T2]1/2
T1 = 27oC
= 300K
T2 = 927oC
= 1200K
V1 = 0.3ms-1
V2 = [T2/T1]1/2 × V1
V1/V2 = [T1/T2]1/2
T1 = 27oC
= 300K
T2 = 927oC
= 1200K
V1 = 0.3ms-1
V2 = [T2/T1]1/2 × V1
= [1200/300] × 0.3
= 0.6ms-1
= 0.6ms-1
A gas will approval ideal behaviour at:- a)Low temperature and low pressure
- b)Low temperature and high pressure
- c)High temperature and low pressure
- d)High temperature and high pressure
Correct answer is option 'C'. Can you explain this answer?
A gas will approval ideal behaviour at:
a)
Low temperature and low pressure
b)
Low temperature and high pressure
c)
High temperature and low pressure
d)
High temperature and high pressure

|
Anirban Khanna answered |
At high temperature and low pressure, the gas volume is infinitely large and both intermolecular force as well as molecular volume can be ignored. Under this condition postulates of kinetic theory applies appropriately and gas approaches ideal behavior.
In an ideal monoatomic gas, the speed of sound is given by 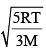 . If the speed of sound in argon at 25°C us 1245 km h–1, the root mean square velocity in ms–1 is…………….
. If the speed of sound in argon at 25°C us 1245 km h–1, the root mean square velocity in ms–1 is…………….
Correct answer is '463'. Can you explain this answer?
In an ideal monoatomic gas, the speed of sound is given by 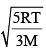 . If the speed of sound in argon at 25°C us 1245 km h–1, the root mean square velocity in ms–1 is…………….
. If the speed of sound in argon at 25°C us 1245 km h–1, the root mean square velocity in ms–1 is…………….

|
Varun Yadav answered |
From kinetic theory of ideal gas,
vs= sqrt[ 5RT/3M]
But,M=mxNo, where m is mass of Argon atom and No is Avogadro number.
Also, R= kNo, where k is Boltzmann constant. Therefore ,
vs=sqrt[ 5 No kT/3mNo]=sqrt[5kT/3m]……(1)
But, (1/2)m<v2 > =(3/2)kT OR
kT=m<v2>/3. Using this in equation (1),
vs= sqrt[5m<v2>/9m]= sqrt[(5/9)<v2] OR
Sqrt(<v2>)=vs x sqrt(9/5)=1245x(5/18)xsqrt(9/5) OR
v rms= 463.9 m/s.
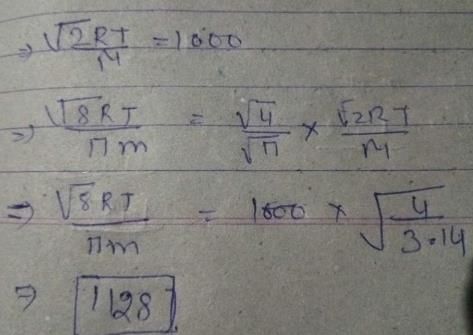
A spherical balloon of 21 cm diameter is to be filled up with hydrogen at NTP from a cylinder containing the gas at 20 atmospheres at 27°C. If the cylinder can hold 2.82 L of water, calculate the number of balloons that can be filled up.
Correct answer is '10'. Can you explain this answer?
A spherical balloon of 21 cm diameter is to be filled up with hydrogen at NTP from a cylinder containing the gas at 20 atmospheres at 27°C. If the cylinder can hold 2.82 L of water, calculate the number of balloons that can be filled up.

|
Srishti Kulkarni answered |

Chapter doubts & questions for Theory of Gases - Physical Chemistry 2025 is part of Chemistry exam preparation. The chapters have been prepared according to the Chemistry exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for Chemistry 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Theory of Gases - Physical Chemistry in English & Hindi are available as part of Chemistry exam.
Download more important topics, notes, lectures and mock test series for Chemistry Exam by signing up for free.
Physical Chemistry
90 videos|144 docs|67 tests
|

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up within 7 days!
Access 1000+ FREE Docs, Videos and Tests
Takes less than 10 seconds to signup