All Exams >
NEET >
4 Months Preparation for NEET >
All Questions
All questions of Equilibrium - Physics for NEET Exam
Ca(HCO3)2 is strongly heated and after equilibrium is attained, temperature changed to 25° C.
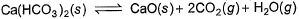
Kp = 36 (pressure taken in atm)
Thus, pressure set up due to CO2 is- a)36 atm
- b)18 atm
- c)12 atm
- d)6 atm
Correct answer is 'D'. Can you explain this answer?
Ca(HCO3)2 is strongly heated and after equilibrium is attained, temperature changed to 25° C.
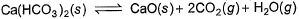
Kp = 36 (pressure taken in atm)
Thus, pressure set up due to CO2 is
Kp = 36 (pressure taken in atm)
Thus, pressure set up due to CO2 is
a)
36 atm
b)
18 atm
c)
12 atm
d)
6 atm
|
|
Mira Joshi answered |
The reaction is as follow:-
Ca(HCO3)2(s)⇌CaO(s) + 2CO2 (g) + H2O(g)
At 25° C H2O goes in liquid state
Kp = (PCaO)1×(PCO2)2
(PCa(HCO3)2)
Since, Ca(HCO3)2, CaO and H2O are not in gaseous state, so their partial pressure is taken 1.
Putting all values, we have
36 = (PCO2)2
Or PCO2 = 6 atm
Ca(HCO3)2(s)⇌CaO(s) + 2CO2 (g) + H2O(g)
At 25° C H2O goes in liquid state
Kp = (PCaO)1×(PCO2)2
(PCa(HCO3)2)
Since, Ca(HCO3)2, CaO and H2O are not in gaseous state, so their partial pressure is taken 1.
Putting all values, we have
36 = (PCO2)2
Or PCO2 = 6 atm
Rate of reaction is proportional to product of molar concentration of reactants with each concentration term raised to power its stoichiometric coefficient. This is the law of- a)Equilibrium
- b)Mass action
- c)Constant proportion
- d)Reciprocal proportions
Correct answer is option 'B'. Can you explain this answer?
Rate of reaction is proportional to product of molar concentration of reactants with each concentration term raised to power its stoichiometric coefficient. This is the law of
a)
Equilibrium
b)
Mass action
c)
Constant proportion
d)
Reciprocal proportions

|
Cutie Pie answered |
Mass action
Following equilibrium is set up at 298 K in a 1 L flask.
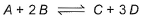
If one starts with 2 moles of A and 1 mole of B, it is found that moles of B and D are equal.Thus Kc is - a)9.0
- b)15.0
- c)3.0
- d)0.0667
Correct answer is option 'B'. Can you explain this answer?
Following equilibrium is set up at 298 K in a 1 L flask.
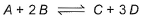
If one starts with 2 moles of A and 1 mole of B, it is found that moles of B and D are equal.Thus Kc is
If one starts with 2 moles of A and 1 mole of B, it is found that moles of B and D are equal.Thus Kc is
a)
9.0
b)
15.0
c)
3.0
d)
0.0667

|
Sushil Kumar answered |
For the equilibrium reaction:
A+2B ⇌ 2C+D
volume of flask = 1L
Initial moles of A = 2 mol
initial concentration of A=[A]i = 2 M
initial mole of B = 1 mol
[B]i = 1 M
[A]eq = 2-x, [B]eq = 1-2x, [C]eq = x, [D]eq = 3x
Given [D]eq = 1 * 1L
= 1 M
Thus x = 1M
[A]eq = 1, [B]eq = -1, [C]eq = 1, [D] = 3
Kc = {([D]eq)3 * ([C]eq)}/{[A]eq * ([B]eq)2}
= Kc = {(3)3*1}/{1*(-1)2}
= 27/1
= 27
A+2B ⇌ 2C+D
volume of flask = 1L
Initial moles of A = 2 mol
initial concentration of A=[A]i = 2 M
initial mole of B = 1 mol
[B]i = 1 M
[A]eq = 2-x, [B]eq = 1-2x, [C]eq = x, [D]eq = 3x
Given [D]eq = 1 * 1L
= 1 M
Thus x = 1M
[A]eq = 1, [B]eq = -1, [C]eq = 1, [D] = 3
Kc = {([D]eq)3 * ([C]eq)}/{[A]eq * ([B]eq)2}
= Kc = {(3)3*1}/{1*(-1)2}
= 27/1
= 27
Which of the following is not a general characteristic of equilibria involving physical processes?- a)Equilibrium is possible only in a closed system at a given temperature.
- b)All measurable properties of the system remain constant.
- c)All the physical processes stop at equilibrium.
- d)The opposing processes occur at the same rate and there is dynamic but stable condition.
Correct answer is option 'C'. Can you explain this answer?
Which of the following is not a general characteristic of equilibria involving physical processes?
a)
Equilibrium is possible only in a closed system at a given temperature.
b)
All measurable properties of the system remain constant.
c)
All the physical processes stop at equilibrium.
d)
The opposing processes occur at the same rate and there is dynamic but stable condition.
|
|
Mira Sharma answered |
All the physical processes like melting of ice and freezing of water, etc., do not stop at equilibrium.
The exothermic formaton of ClF3 is represented by the equation -Cl2(g)+3F2(g)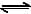 2ClF3(g) ; ΔrH = -329 kJWhich of the following will increase the quantity of ClF3 in an equilibrium mixture of Cl2, F2 and ClF3 ?
2ClF3(g) ; ΔrH = -329 kJWhich of the following will increase the quantity of ClF3 in an equilibrium mixture of Cl2, F2 and ClF3 ?- a)Removing Cl2
- b)Increasing the temperature
- c)Adding F2
- d)Increasing the volume of the container
Correct answer is option 'C'. Can you explain this answer?
The exothermic formaton of ClF3 is represented by the equation -
Cl2(g)+3F2(g)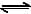 2ClF3(g) ; ΔrH = -329 kJ
2ClF3(g) ; ΔrH = -329 kJ
Which of the following will increase the quantity of ClF3 in an equilibrium mixture of Cl2, F2 and ClF3 ?
a)
Removing Cl2
b)
Increasing the temperature
c)
Adding F2
d)
Increasing the volume of the container
|
|
Hansa Sharma answered |
The correct answer is option C
According to Le-Chatelier's Principle, if a system at equilibrium is subjected to a change of concentration pressure or temperature then the equilibrium is shifted in such a way as to nullify the effect of change.
In the given reaction, if the concentration of F2 is increased then the reaction will shift in the forward direction in order to increase the concentration of ClF3.
Hence,
Adding F2 .
According to Le-Chatelier's Principle, if a system at equilibrium is subjected to a change of concentration pressure or temperature then the equilibrium is shifted in such a way as to nullify the effect of change.
In the given reaction, if the concentration of F2 is increased then the reaction will shift in the forward direction in order to increase the concentration of ClF3.
Hence,
Adding F2 .
Graphite is added to a vessel that contains CO2(g) at a pressure of 0.830 atm at a certain high temperature. The pressure rises due to a reaction that produces CO (g). The total pressure reaches an equilibrium value of 1.366 atm. Calculate the equilibrium constant of the following reaction.
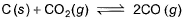
- a)2.909 atm
- b)6.909 atm
- c)4.909 atm
- d)3.909 atm
Correct answer is option 'D'. Can you explain this answer?
Graphite is added to a vessel that contains CO2(g) at a pressure of 0.830 atm at a certain high temperature. The pressure rises due to a reaction that produces CO (g). The total pressure reaches an equilibrium value of 1.366 atm. Calculate the equilibrium constant of the following reaction.
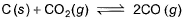
a)
2.909 atm
b)
6.909 atm
c)
4.909 atm
d)
3.909 atm
|
|
Hansa Sharma answered |

How many of the following are Lewis bases?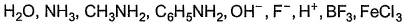
Correct answer is '6'. Can you explain this answer?
How many of the following are Lewis bases?
|
|
Pooja Shah answered |
Total number of compounds acting as Lewis base in the given example is 6. H+ and BF3 are electron deficient so they can't act as Lewis base while FeCl3 acts as Lewis acid so all the other compounds except these three are Lewis base.
In which of the following reaction can equilibrium be attained- a)Reversible reaction
- b)Cyclic reaction
- c)Decomposition reaction
- d)Irreversible reaction
Correct answer is option 'A'. Can you explain this answer?
In which of the following reaction can equilibrium be attained
a)
Reversible reaction
b)
Cyclic reaction
c)
Decomposition reaction
d)
Irreversible reaction
|
|
Rajat Kapoor answered |
Reversible Reaction
The common observation for any reactions when they are reacted in closed containers would not go to completion, for some given conditions like temperature and pressure.
For all those cases, only the reactants are found to be present in the intial stages, but with the progress of reaction, the reactants concentration decreases and to that of the products increases. A stage is finally reached where there is no more change of reactants and products concentration is observed. The state where the reactants and products concentrations do not show any visible change within a given period of time is better known as the state of chemical equilibrium.
The reactant amount that remains unused depends upon the experimental conditions like concentration of components, temperature of the system, pressure of the system and the reaction nature.
Direction (Q. Nos. 1-10) This section contains 10 multiple choice questions. Each question has four
choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.Q. Vapour pressure of NH4HS (s)is 20 mm at 25°C, for
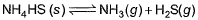
Total pressure when NH4HS (s) dissociates at 25°Cin a vessel which already contains H2S (g)at a pressure of 15 mm, is- a)25 mm
- b)50 mm
- c)5 mm
- d)10 mm
Correct answer is option 'A'. Can you explain this answer?
Direction (Q. Nos. 1-10) This section contains 10 multiple choice questions. Each question has four
choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Q. Vapour pressure of NH4HS (s)is 20 mm at 25°C, for
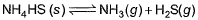
Total pressure when NH4HS (s) dissociates at 25°Cin a vessel which already contains H2S (g)at a pressure of 15 mm, is
Total pressure when NH4HS (s) dissociates at 25°Cin a vessel which already contains H2S (g)at a pressure of 15 mm, is
a)
25 mm
b)
50 mm
c)
5 mm
d)
10 mm
|
|
Hansa Sharma answered |
The correct answer is Option A.
NH4HS -------> NH3 + H2S
Let P be the pressure at eq. of NH3 and H2S.
Therefore, Kp = P2
= (20 / 2)2
= 100 mm
= 100
Also, Kp = (15 + P) (P)
100 = 15 P + P2
P2 + 15 P – 100 = 0
P = 5
Total pressure = 15 + 2(P)
= 15 + 2(5)
= 25 mm
NH4HS -------> NH3 + H2S
Let P be the pressure at eq. of NH3 and H2S.
Therefore, Kp = P2
= (20 / 2)2
= 100 mm
= 100
Also, Kp = (15 + P) (P)
100 = 15 P + P2
P2 + 15 P – 100 = 0
P = 5
Total pressure = 15 + 2(P)
= 15 + 2(5)
= 25 mm
For the reaction 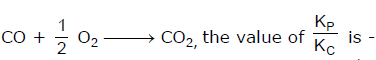 [AIEEE-2002]
[AIEEE-2002]- a)
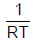
- b)

- c)
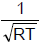
- d)RT
Correct answer is option 'C'. Can you explain this answer?
For the reaction 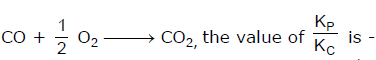
[AIEEE-2002]
a)
b)
c)
d)
RT
|
|
Raghav Bansal answered |
The correct answer is option C
Kp = Kc (RT)Δn;
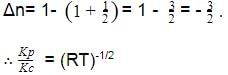
Kp = Kc (RT)Δn;
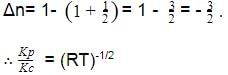
Following equilibrium is set up at 1000 K and 1 bar in a 5 L flask,
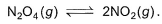
At equilibrium, NO2 is 50% o f the total volume. Thus, equilibrium constant Kc is - a)0.133
- b)0.266
- c)0.200
- d)0.400
Correct answer is option 'A'. Can you explain this answer?
Following equilibrium is set up at 1000 K and 1 bar in a 5 L flask,
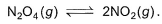
At equilibrium, NO2 is 50% o f the total volume. Thus, equilibrium constant Kc is
At equilibrium, NO2 is 50% o f the total volume. Thus, equilibrium constant Kc is
a)
0.133
b)
0.266
c)
0.200
d)
0.400

|
Manish Aggarwal answered |
The correct answer is Option A.
N2O4 ⇌ 2NO2
Initial 1 0
Equilibrium 1−x 2x
N2O4 ⇌ 2NO2
Initial 1 0
Equilibrium 1−x 2x
Total moles = 1 - x + 2x
NO2 is 50% of the total volume when equilibrium is set up.
Thus, the volume fraction (at equilibrium) of NO2 = 50/100 = 0.5 = ½
So, 2x / (1+x) = ½
=> x = ⅓
Thus, the volume fraction (at equilibrium) of NO2 = 50/100 = 0.5 = ½
So, 2x / (1+x) = ½
=> x = ⅓
For 1 litre;
Kc = [NO2] / [N2O4]
= [4*(1/9)] / [⅔]
= 0.66;
Kc = [NO2] / [N2O4]
= [4*(1/9)] / [⅔]
= 0.66;
For 5 litres;
Kc = 0.66 / 5
= 0.133
Thus, option A is correct.
Kc = 0.66 / 5
= 0.133
Thus, option A is correct.
Assume following equilibria when total pressure set up in each are equal to 1 atm, and equilibrium constant (Kp) as K1; K2 and K3
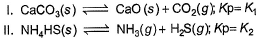
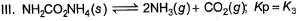
Thus,- a) K1 = K2 = K3
- b)K1 < K2 < K3
- c)K3 < K2 < K1
- d)None of these
Correct answer is option 'C'. Can you explain this answer?
Assume following equilibria when total pressure set up in each are equal to 1 atm, and equilibrium constant (Kp) as K1; K2 and K3
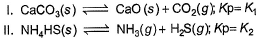
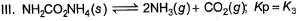
Thus,
Thus,
a)
K1 = K2 = K3
b)
K1 < K2 < K3
c)
K3 < K2 < K1
d)
None of these

|
Pioneer Academy answered |
The correct answer is option C
CaCO3 → CaO + CO2
Kp = k1 = Pco2
total pressure of container P
k1 = p
NH4HS → NH3 + H2S
PNH3 = PH2S = P0
P0 + P0 = p (total pressure)
P0 = p/2
k2 = kp = [PNH3][PH2s] p24
NH2CoNH2 → 2NH3 + CO2
PNH3 = 2P0 PCO2 = P0
2P0 + P0 = P
CaCO3 → CaO + CO2
Kp = k1 = Pco2
total pressure of container P
k1 = p
NH4HS → NH3 + H2S
PNH3 = PH2S = P0
P0 + P0 = p (total pressure)
P0 = p/2
k2 = kp = [PNH3][PH2s] p24
NH2CoNH2 → 2NH3 + CO2
PNH3 = 2P0 PCO2 = P0
2P0 + P0 = P
At 1000 K, pressure of CO2 in equilibrium with CaCO3 and CaO is equal to 2.105 atm. The equilibrium constant for the reaction,
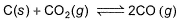
is 1.9 at the same temperature when pressure are in atm. Solid C, CaO, and CaCO3 are mixed and allowed to come to equilibrium at 1000 K in a closed vessel.Q. What is the pressure of CO (g)at equilibrium (in atm)?
Correct answer is '2'. Can you explain this answer?
At 1000 K, pressure of CO2 in equilibrium with CaCO3 and CaO is equal to 2.105 atm. The equilibrium constant for the reaction,
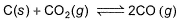
is 1.9 at the same temperature when pressure are in atm. Solid C, CaO, and CaCO3 are mixed and allowed to come to equilibrium at 1000 K in a closed vessel.
is 1.9 at the same temperature when pressure are in atm. Solid C, CaO, and CaCO3 are mixed and allowed to come to equilibrium at 1000 K in a closed vessel.
Q. What is the pressure of CO (g)at equilibrium (in atm)?

|
Learners Habitat answered |
K= (partial pressure of co2/(partial pressure of co2)
since k =1.9
So 1.9 = (partial pressure of co)2/2.105
(partial pressure of co)2 =2.105×1.9
= 3.99 = 4
(partial pressure of co) =2
since k =1.9
So 1.9 = (partial pressure of co)2/2.105
(partial pressure of co)2 =2.105×1.9
= 3.99 = 4
(partial pressure of co) =2
Ca(HCO3)2 decomposes as,
Ca (HCO3)2(s) ⇌ CaCO3(s) + H2O(g) + CO2(g)
Equilibrium pressure is found to be 0.12 bar. What is pco2 if the reaction mixture also contains H2O(g)at 0.20 bar?
- a) 0.20 bar
- b) 0.017 ba
- c)0.040 bar
- d)0.10 bar
Correct answer is option 'B'. Can you explain this answer?
Ca(HCO3)2 decomposes as,
Ca (HCO3)2(s) ⇌ CaCO3(s) + H2O(g) + CO2(g)
Equilibrium pressure is found to be 0.12 bar. What is pco2 if the reaction mixture also contains H2O(g)at 0.20 bar?
Equilibrium pressure is found to be 0.12 bar. What is pco2 if the reaction mixture also contains H2O(g)at 0.20 bar?
a)
0.20 bar
b)
0.017 ba
c)
0.040 bar
d)
0.10 bar

|
Top Rankers answered |

The equilibrium constant for the following reaction, is 1.6 x 105 at 1024 K.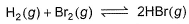
HBr (g)at 10.0 bar is introduced into a sealed container at 1024 K. Thus, partial pressure of H2(g)and Br2(g), together is- a)10 bar
- b)0.05 bar
- c)0.025 bar
- d)0.10 bar
Correct answer is option 'A'. Can you explain this answer?
The equilibrium constant for the following reaction, is 1.6 x 105 at 1024 K.
HBr (g)at 10.0 bar is introduced into a sealed container at 1024 K. Thus, partial pressure of H2(g)and Br2(g), together is
a)
10 bar
b)
0.05 bar
c)
0.025 bar
d)
0.10 bar
|
|
Rahul Bansal answered |
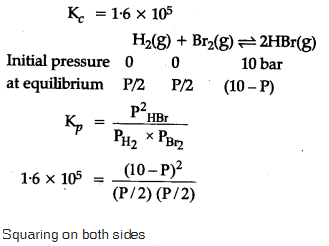
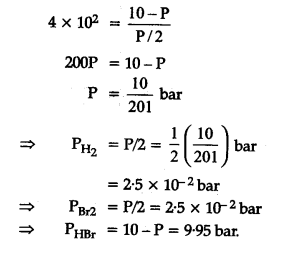
=> 10 bar approximately
For the reversible reaction, 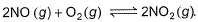
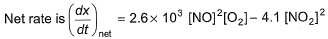
In a reaction vessel, [NO]= [O2]= 0.01 mol L-1 and [NO2]= 0.1 mol L-1 then above reaction is - a)shifted in forward direction
- b)shifted in backward direction
- c)in equilibrium
- d)not predictable in the absence of required data
Correct answer is option 'A'. Can you explain this answer?
For the reversible reaction, 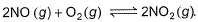
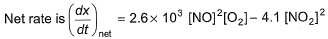
In a reaction vessel, [NO]= [O2]= 0.01 mol L-1 and [NO2]= 0.1 mol L-1 then above reaction is
In a reaction vessel, [NO]= [O2]= 0.01 mol L-1 and [NO2]= 0.1 mol L-1 then above reaction is
a)
shifted in forward direction
b)
shifted in backward direction
c)
in equilibrium
d)
not predictable in the absence of required data
|
|
Lavanya Menon answered |
On substituting the values of conc. of NO, O2 and NO2 in given rate equation, we get a +ve (positive) value indicating that the reaction takes place in forward direction.
A sample of N2O4(g)with a pressure of 1.00 atm is placed in a flask. When equilibrium is reached, 20% of N2O4(g)has been converted to NO2(g)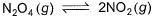 If the original pressure is made 10% of the earlier pressure, then per cent dissociation will be
If the original pressure is made 10% of the earlier pressure, then per cent dissociation will be- a)20%
- b)42%
- c)54%
- d)62%
Correct answer is option 'C'. Can you explain this answer?
A sample of N2O4(g)with a pressure of 1.00 atm is placed in a flask. When equilibrium is reached, 20% of N2O4(g)has been converted to NO2(g)
If the original pressure is made 10% of the earlier pressure, then per cent dissociation will be
a)
20%
b)
42%
c)
54%
d)
62%

|
Lohit Matani answered |
Correct answer is A.

In the following reaction,
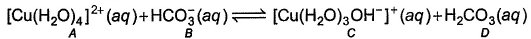
Species behaving as Bronsted-Lowry acids are- a)A,D
- b)B,C
- c)A,B
- d)B,D
Correct answer is option 'A'. Can you explain this answer?
In the following reaction,
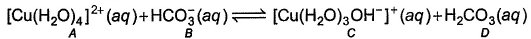
Species behaving as Bronsted-Lowry acids are
Species behaving as Bronsted-Lowry acids are
a)
A,D
b)
B,C
c)
A,B
d)
B,D
|
|
Om Desai answered |
Bronsted lowry acids are those acids which donate H+. In the reaction, A and D are giving H+. So, these both are bronsted lowry acid.
Kc forthe decomposition of NH4HS(s) is 1.8x 10-4 at 25°C.
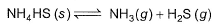
If the system already contains [NH3] = 0.020 M, then when equilibrium is reached, molar concentration are
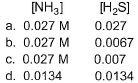
- a)a
- b)b
- c)c
- d)d
Correct answer is option 'B'. Can you explain this answer?
Kc forthe decomposition of NH4HS(s) is 1.8x 10-4 at 25°C.
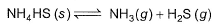
If the system already contains [NH3] = 0.020 M, then when equilibrium is reached, molar concentration are

If the system already contains [NH3] = 0.020 M, then when equilibrium is reached, molar concentration are
a)
a
b)
b
c)
c
d)
d

|
Sushil Kumar answered |
NH4HS (s) ⇋ NH3 (g) + H2S (g)
Initial 1 - -
At eqm 1-x x+0.02 x
Kc = [NH3][H2S] (Since NH4HS is solid, we ignore it.)
1.8×10-4 = (x+0.02)(x)
x2+0.02x-1.8×10-4 = 0
Applying quadratic formula; x = -0.02+√{(0.02)2-4×1.8×10-4}
= 0.033-0.020/2 = 0.0065
Therefore, concn of NH3 at equilibrium = x+0.020 = 0.0265
concn of H2S at equilibrium = x = 0.0065
So, option b is correct
Initial 1 - -
At eqm 1-x x+0.02 x
Kc = [NH3][H2S] (Since NH4HS is solid, we ignore it.)
1.8×10-4 = (x+0.02)(x)
x2+0.02x-1.8×10-4 = 0
Applying quadratic formula; x = -0.02+√{(0.02)2-4×1.8×10-4}
= 0.033-0.020/2 = 0.0065
Therefore, concn of NH3 at equilibrium = x+0.020 = 0.0265
concn of H2S at equilibrium = x = 0.0065
So, option b is correct
Equilibrium constant for the reaction PCL5⇋PCL3+CL2 is 0.0205 at 230°C and 1 atmospheric pressure if at equilibrium concentration of PCL5 is 0.235 moles liter−1liter-1and that of CL2= 0.028 moleslit−1lit-1 then conc. of PCL3 at equilibrium is- a)0.0174
- b)0.174
- c)0.0348
- d)1.74
Correct answer is option 'A'. Can you explain this answer?
Equilibrium constant for the reaction PCL5⇋PCL3+CL2 is 0.0205 at 230°C and 1 atmospheric pressure if at equilibrium concentration of PCL5 is 0.235 moles liter−1liter-1and that of CL2= 0.028 moleslit−1lit-1 then conc. of PCL3 at equilibrium is
a)
0.0174
b)
0.174
c)
0.0348
d)
1.74
|
|
Mira Sharma answered |

Phosphorus pentachloride dissociates as follows, is a closed reaction vessel,PCl5(g) 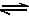 PCl3(g) + Cl2(g)If total pressure at equilibrium of the reaction mixture is P and degree of dissociation of PCl5 is x, the partial pressure of PCl3 will be [AIEEE 2006]
PCl3(g) + Cl2(g)If total pressure at equilibrium of the reaction mixture is P and degree of dissociation of PCl5 is x, the partial pressure of PCl3 will be [AIEEE 2006]- a)
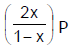
- b)
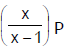
- c)
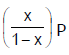
- d)
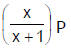
Correct answer is option 'D'. Can you explain this answer?
Phosphorus pentachloride dissociates as follows, is a closed reaction vessel,
PCl5(g) 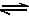 PCl3(g) + Cl2(g)
PCl3(g) + Cl2(g)
If total pressure at equilibrium of the reaction mixture is P and degree of dissociation of PCl5 is x, the partial pressure of PCl3 will be
[AIEEE 2006]
a)
b)
c)
d)
|
|
Gaurav Kumar answered |
Which of the following equilibria is not affected by change in volume of the flask [AIEEE-2002]- a)PCl5 (g)
 PCl3(g) + Cl2(g)
PCl3(g) + Cl2(g) - b)N2(g) + 3H2 (g)
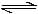 2NH3(g)
2NH3(g) - c)N2 (g) + O2 (g)
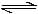 2NO (g)
2NO (g) - d)SO2Cl2 (g)
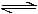 SO2(g) + Cl2(g)
SO2(g) + Cl2(g)
Correct answer is option 'C'. Can you explain this answer?
Which of the following equilibria is not affected by change in volume of the flask [AIEEE-2002]
a)
PCl5 (g) 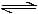 PCl3(g) + Cl2(g)
PCl3(g) + Cl2(g)
b)
N2(g) + 3H2 (g) 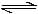 2NH3(g)
2NH3(g)
c)
N2 (g) + O2 (g) 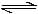 2NO (g)
2NO (g)
d)
SO2Cl2 (g) 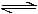 SO2(g) + Cl2(g)
SO2(g) + Cl2(g)
|
|
Gaurav Kumar answered |
Change in volume affects number of moles per unit volume and move in the direction which undo the change.
N2(g)+ O2(g) ⇄2NO(g)
Number of moles of reactants and products are equal.
Passage ISolid ammonium chloride is in equilibrium with ammonia and hydrogen chloride gases
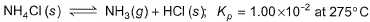
0.980 g of solid NH4CI is taken in a closed vessel of 1 L capacity and heated to 275° C.Q. Percentage decomposition of the original sample is- a)24.81%
- b)6.24%
- c)3.12%
- d)12.13%
Correct answer is option 'D'. Can you explain this answer?
Passage I
Solid ammonium chloride is in equilibrium with ammonia and hydrogen chloride gases
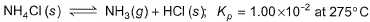
0.980 g of solid NH4CI is taken in a closed vessel of 1 L capacity and heated to 275° C.
0.980 g of solid NH4CI is taken in a closed vessel of 1 L capacity and heated to 275° C.
Q. Percentage decomposition of the original sample is
a)
24.81%
b)
6.24%
c)
3.12%
d)
12.13%

|
Knowledge Hub answered |
The state of HCl is given wrong. It will be in gaseous state.
So, the reaction be like;-
NH4Cl(s) ⇌ NH3(g) + HCl(g) kp = 1.00×10-2 at 275° C
Kp = kc(RT)2
1.00×10-2 = kc(0.0821×548)2
Or kc = 4.94×10-6
NH4Cl(s) ⇌ NH3(g) + HCl(g)
Initial 1 - -
At eqm 1-x x x
Kc = x2
x = √(4.94×10-6)
= 2.22×10-3
Therefore, NH4Cl dissociated at eqm = 2.22×10-3 × 53.5 = 0.118
%age decomposition = 0.118/0.980×100 = 12.13%
So, the reaction be like;-
NH4Cl(s) ⇌ NH3(g) + HCl(g) kp = 1.00×10-2 at 275° C
Kp = kc(RT)2
1.00×10-2 = kc(0.0821×548)2
Or kc = 4.94×10-6
NH4Cl(s) ⇌ NH3(g) + HCl(g)
Initial 1 - -
At eqm 1-x x x
Kc = x2
x = √(4.94×10-6)
= 2.22×10-3
Therefore, NH4Cl dissociated at eqm = 2.22×10-3 × 53.5 = 0.118
%age decomposition = 0.118/0.980×100 = 12.13%
In which of the following solvents is silver chloride most soluble?- a)0.1 mol dm–3 AgNO3 solution
- b)0.1 mol dm–3 HCl solution
- c)H2O
- d)Aqueous ammonia
Correct answer is option 'D'. Can you explain this answer?
In which of the following solvents is silver chloride most soluble?
a)
0.1 mol dm–3 AgNO3 solution
b)
0.1 mol dm–3 HCl solution
c)
H2O
d)
Aqueous ammonia
|
|
Naina Bansal answered |
Silver chloride forms a soluble complex with aqueous ammonia.
AgCl + 2NH3→ [Ag(NH3)2]Cl
Direction (Q. Nos. 21) This section contains 2 questions. when worked out will result in an integer from 0 to 9 (both inclusive)Q. For the equilibrium in gaseous phase in 2 L flask we start with 2 moles of SO2 and 1 mole of O2 at 3 atm, 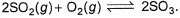
When equilibrium is attained, pressure changes to 2.5 atm. Hence, equilibrium constant Kc is
Correct answer is '4'. Can you explain this answer?
Direction (Q. Nos. 21) This section contains 2 questions. when worked out will result in an integer from 0 to 9 (both inclusive)
Q. For the equilibrium in gaseous phase in 2 L flask we start with 2 moles of SO2 and 1 mole of O2 at 3 atm, 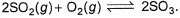
When equilibrium is attained, pressure changes to 2.5 atm. Hence, equilibrium constant Kc is
When equilibrium is attained, pressure changes to 2.5 atm. Hence, equilibrium constant Kc is
|
|
Om Desai answered |
The correct answer is 4
2SO2(g) + O2(g) ⇋ 2SO3
Initial moles 2 1
At equilibrium 2 - 2x 1 - x 2x
Net moles at equilibrium = 2 - 2x + 1 - x + 2x
=(3 - x)moles
Initial:
moles = 3,
Pressure = 3 atm,
Volume = 2L,
PV = nRT
3 x 2 = 3RT -------- 1
At equilibrium
Moles = 3 - x,
Pressure = 2.5 atm
Volume = 2L
P‘V = n’RT ---------- 2
Divide eqn 2 by 1
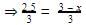
⇒2.5 = 3 - x
⇒x = 0.5
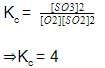
2SO2(g) + O2(g) ⇋ 2SO3
Initial moles 2 1
At equilibrium 2 - 2x 1 - x 2x
Net moles at equilibrium = 2 - 2x + 1 - x + 2x
=(3 - x)moles
Initial:
moles = 3,
Pressure = 3 atm,
Volume = 2L,
PV = nRT
3 x 2 = 3RT -------- 1
At equilibrium
Moles = 3 - x,
Pressure = 2.5 atm
Volume = 2L
P‘V = n’RT ---------- 2
Divide eqn 2 by 1
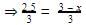
⇒2.5 = 3 - x
⇒x = 0.5
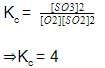
For the equilibrium,
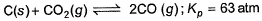
at 1000 K. If at equilibrium pCO = 10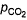 then total pressure at equilibrium is
then total pressure at equilibrium is - a)6.30 atm
- b)0.63 atm
- c)6.93 atm
- d)69.3 atm
Correct answer is option 'C'. Can you explain this answer?
For the equilibrium,
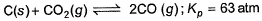
at 1000 K. If at equilibrium pCO = 10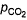 then total pressure at equilibrium is
then total pressure at equilibrium is
at 1000 K. If at equilibrium pCO = 10
a)
6.30 atm
b)
0.63 atm
c)
6.93 atm
d)
69.3 atm
|
|
Lavanya Menon answered |
C(s) + CO2(g) <=========> 2CO(g)
Kp = pCO2/pCO2
GIven Kp = 63 and pCO = 10pCO2
Putting the value of pCO in above equation,
63 = 100(pCO2)2/pCO2
Or pCO2 = 0.63
pCO = 6.3
Therefore, total pressure = 6.3+0.63 = 6.93 atm
Kp = pCO2/pCO2
GIven Kp = 63 and pCO = 10pCO2
Putting the value of pCO in above equation,
63 = 100(pCO2)2/pCO2
Or pCO2 = 0.63
pCO = 6.3
Therefore, total pressure = 6.3+0.63 = 6.93 atm
Equilibrium can be attained i- a)all types of system
- b)closed system
- c)open system
- d)isolated system
Correct answer is option 'B'. Can you explain this answer?
Equilibrium can be attained i
a)
all types of system
b)
closed system
c)
open system
d)
isolated system
|
|
Preeti Iyer answered |
The equilibrium state can only be reached if the chemical reaction takes place in a closed system. Otherwise, some of the products may escape, leading to the absence of a reverse reaction. (Note that in the diagrams under "Characteristics of Chemical Equilibrium," all reactions are in closed systems.)
Can you explain the answer of this question below:Equilibrium reactions are found in large scale in production of
- A:
ammonia
- B:
sulfuric acid
- C:
lactic acid
- D:
both A and B
The answer is d.
Equilibrium reactions are found in large scale in production of
ammonia
sulfuric acid
lactic acid
both A and B
|
|
Shreya Gupta answered |
An understanding of equilibrium is important in the chemical industry. Equilibrium reactions are involved in some of the stages in the large-scale production of ammonia, sulfuric acid and many other chemicals.
For the reaction 2 NO2(g) 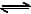 2 NO(g) + O2(g),(Kc = 1.8 × 10_6 at 184ºC)(R = 0.0831 kJ/(mol.K))When Kp and Kc are compared at 184ºC it is found that [AIEEE-2005]
2 NO(g) + O2(g),(Kc = 1.8 × 10_6 at 184ºC)(R = 0.0831 kJ/(mol.K))When Kp and Kc are compared at 184ºC it is found that [AIEEE-2005]- a)Kp is less than Kc
- b)Kp is greater than Kc
- c)Whether Kp is greater than, less than or equal to Kc depends upon the total gas pressure
- d)Kp = Kc
Correct answer is option 'B'. Can you explain this answer?
For the reaction 2 NO2(g) 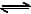 2 NO(g) + O2(g),
2 NO(g) + O2(g),
(Kc = 1.8 × 10_6 at 184ºC)
(R = 0.0831 kJ/(mol.K))
When Kp and Kc are compared at 184ºC it is found that [AIEEE-2005]
a)
Kp is less than Kc
b)
Kp is greater than Kc
c)
Whether Kp is greater than, less than or equal to Kc depends upon the total gas pressure
d)
Kp = Kc
|
|
Gaurav Kumar answered |
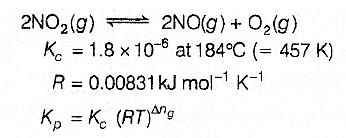
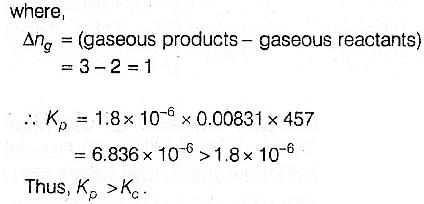
What is the equilibrium expression for the reaction P4(S) + 5O2(g) 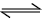 P4O10(s) ? [AIEEE-2004]
P4O10(s) ? [AIEEE-2004]- a)KC = [P4O10] / [P4] [O2]5
- b)KC = [P4O10] / 5 [P4] [O2]
- c)KC = [O2]5
- d)KC = 1/ [O2]5
Correct answer is option 'D'. Can you explain this answer?
What is the equilibrium expression for the reaction P4(S) + 5O2(g) 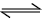 P4O10(s) ?
P4O10(s) ?
[AIEEE-2004]
a)
KC = [P4O10] / [P4] [O2]5
b)
KC = [P4O10] / 5 [P4] [O2]
c)
KC = [O2]5
d)
KC = 1/ [O2]5

|
Sravya Banerjee answered |
In the expression for equilibrium constant (Kp or Kc) species state are not written (i.e., their molar concentrations are not taken as 1)
P4 (s) + 5O2 (g) ⇌ P4O10 (s)
Thus, Kc = 1/[O2]5
P4 (s) + 5O2 (g) ⇌ P4O10 (s)
Thus, Kc = 1/[O2]5
At 700 K and 350 bar, a 1 : 3 mixture of N2(g) and H2(g) reacts to form an equilibrium mixture containing X (NH3)= 0.50. Assuming ideal behaviour Kp for the equilibrium reaction, 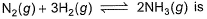
- a)2.03x 10-4
- b)3.55x 10-3
- c)1.02 x 10-4
- d)3.1 x 10-4
Correct answer is option 'D'. Can you explain this answer?
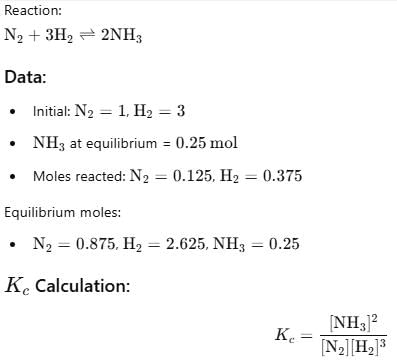
At 700 K and 350 bar, a 1 : 3 mixture of N2(g) and H2(g) reacts to form an equilibrium mixture containing X (NH3)= 0.50. Assuming ideal behaviour Kp for the equilibrium reaction, 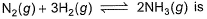
a)
2.03x 10-4
b)
3.55x 10-3
c)
1.02 x 10-4
d)
3.1 x 10-4

|
Knowledge Hub answered |
The correct answer is option A
2.03x 10-4
The given equation is :-
N2(g)+3H2(g) ⇌ 2NH3(g)
Initial moles : 1 3 0
At eqm ; (1−x) (3−3x) (2x)
(let)
Total moles of equation
=1 − x + 3 − 3x + 2x = (4−2x)
Now, X(NH3) =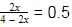
⇒ 2x = 2 − x
⇒ 3x = 2 ⇒ x = 0.66 =
32
Now, at equation, moles of N2= 1/3, moles of NH3 = 4/3
moles of H2 =3 − 2 = 1
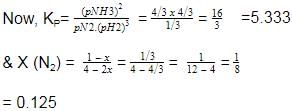
2.03x 10-4
The given equation is :-
N2(g)+3H2(g) ⇌ 2NH3(g)
Initial moles : 1 3 0
At eqm ; (1−x) (3−3x) (2x)
(let)
Total moles of equation
=1 − x + 3 − 3x + 2x = (4−2x)
Now, X(NH3) =
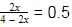
⇒ 2x = 2 − x
⇒ 3x = 2 ⇒ x = 0.66 =
32
Now, at equation, moles of N2= 1/3, moles of NH3 = 4/3
moles of H2 =3 − 2 = 1
PH can be kept constant with help of- a)saturated solution
- b)unsaturated solution
- c)buffer solution
- d)super saturated solution
Correct answer is option 'C'. Can you explain this answer?
PH can be kept constant with help of
a)
saturated solution
b)
unsaturated solution
c)
buffer solution
d)
super saturated solution
|
|
Nandini Iyer answered |
A buffer is an aqueous solution containing a weak acid and its conjugate base or a weak base and its conjugate acid. ... It is used to prevent any change in the pH of a solution, regardless of solute. Buffer solutions are used as a means of keeping pH at a nearly constant value in a wide variety of chemical applications.
Direction (Q. Nos. 1-15) This section contains 15 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.Q. Which of the following is least likely to behave as Lewis base?- a)OH-
- b)H2O
- c)NH3
- d)BF3
Correct answer is option 'D'. Can you explain this answer?
Direction (Q. Nos. 1-15) This section contains 15 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Q. Which of the following is least likely to behave as Lewis base?
a)
OH-
b)
H2O
c)
NH3
d)
BF3

|
Savita Soni answered |
Because it is a electron deficient compound thats why it will behave like a lewis acid not like a lewis base.
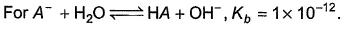
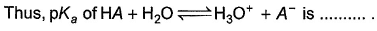
Correct answer is '2'. Can you explain this answer?
|
|
Suresh Reddy answered |
The correct answer is 2
Here
we know [OH-][H+]=10-14 at 25 degree Celsius
so pka+pkb=14
hence answer is 2
Here
we know [OH-][H+]=10-14 at 25 degree Celsius
so pka+pkb=14
hence answer is 2
Direction (Q. Nos. 16-19) This section contains a paragraph, each describing theory, experiments, data etc. three Questions related to paragraph have been given.Each question have only one correct answer among the four given options (a),(b),(c),(d)Passage I
The equilibrium reaction 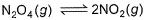 has been thoroughly studied Kp = 0.148 at 298 KIf the total pressure in a flask containing NO2 and N2O4 gas at 25°C is 1.50 atm, what fraction of the N2O4 has dissociated to NO2 ?
has been thoroughly studied Kp = 0.148 at 298 KIf the total pressure in a flask containing NO2 and N2O4 gas at 25°C is 1.50 atm, what fraction of the N2O4 has dissociated to NO2 ? - a)0.156
- b)0.844
- c)0.024
- d)0.076
Correct answer is option 'A'. Can you explain this answer?
Direction (Q. Nos. 16-19) This section contains a paragraph, each describing theory, experiments, data etc. three Questions related to paragraph have been given.Each question have only one correct answer among the four given options (a),(b),(c),(d)
Passage I
The equilibrium reaction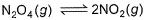 has been thoroughly studied Kp = 0.148 at 298 K
has been thoroughly studied Kp = 0.148 at 298 K
The equilibrium reaction
If the total pressure in a flask containing NO2 and N2O4 gas at 25°C is 1.50 atm, what fraction of the N2O4 has dissociated to NO2 ?
a)
0.156
b)
0.844
c)
0.024
d)
0.076
|
|
Suresh Iyer answered |
The correct answer is Option A.
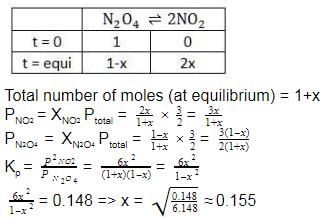
Fraction of N2O4 dissociated = x = 0.155 (x = mole fraction)
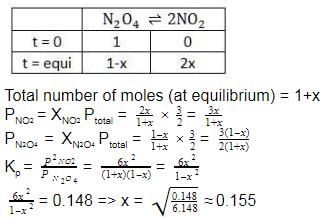
Fraction of N2O4 dissociated = x = 0.155 (x = mole fraction)
At 273 K and 1 atm, 1 L of N2O4 (g) decomposes to NO2(g)a s given,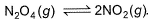 At equilibrium, original volume is 25% less than the existing volume. Percentage decomposition of N2O4 (g) is thus,
At equilibrium, original volume is 25% less than the existing volume. Percentage decomposition of N2O4 (g) is thus, - a)25%
- b)50%
- c)66.66%
- d)33.33%
Correct answer is option 'D'. Can you explain this answer?
At 273 K and 1 atm, 1 L of N2O4 (g) decomposes to NO2(g)a s given,
At equilibrium, original volume is 25% less than the existing volume. Percentage decomposition of N2O4 (g) is thus,
a)
25%
b)
50%
c)
66.66%
d)
33.33%
|
|
Suresh Reddy answered |
Let the initial volume of N2O4 be x and initial volume of NO2 is 0
If the degree of dissociation is a, then the final volume of N2O4 is x(1−a) and NO2 is 2ax.
Initial
It equilibrium
N2O4 ⟶ 2NO2
x 0
x(1−a) 2ax
Total initial volume =x+0=x
Final volume =x(1−a)+2ax=x+ax=x(1+a)
It is given that the initial volume is 25% less than the final volume
x=0.75×(1+a)
1+a=1.33
a=0.33
So %age dissociation = 33.33%
If the degree of dissociation is a, then the final volume of N2O4 is x(1−a) and NO2 is 2ax.
Initial
It equilibrium
N2O4 ⟶ 2NO2
x 0
x(1−a) 2ax
Total initial volume =x+0=x
Final volume =x(1−a)+2ax=x+ax=x(1+a)
It is given that the initial volume is 25% less than the final volume
x=0.75×(1+a)
1+a=1.33
a=0.33
So %age dissociation = 33.33%
Passage lIOne of the reactions that takes place in producing steel from iron ore is the reduction of iron (II) oxide by carbon monoxide to give iron metal and carbon dioxide.
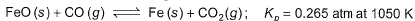
Initial partial pressure
CO(g) = 1.40 atm
CO2(g) = 0.80 atmQ. Under the given partial pressure, reaction is- a)displaced in forward side
- b)displaced in backward side
- c)in equilibrium
- d)incomplete in the absence of total pressure
Correct answer is option 'C'. Can you explain this answer?
Passage lI
One of the reactions that takes place in producing steel from iron ore is the reduction of iron (II) oxide by carbon monoxide to give iron metal and carbon dioxide.
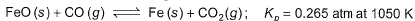
Initial partial pressure
CO(g) = 1.40 atm
CO2(g) = 0.80 atm
Initial partial pressure
CO(g) = 1.40 atm
CO2(g) = 0.80 atm
Q. Under the given partial pressure, reaction is
a)
displaced in forward side
b)
displaced in backward side
c)
in equilibrium
d)
incomplete in the absence of total pressure
|
|
Preeti Iyer answered |
We know that with increase in pressure on one side, reaction shifts to that side which has less no. of moles(of gaseous species). However the no. of moles are same on both sides. So the REACTION WILL REMAIN IN EQUILIBRIUM.
Ammonium carbamate dissociates as,
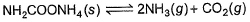
In a closed vessel containing ammonium carbamate in equilibrium with its vapour, ammonia is added such that partial pressure of NH3 now equals the original total pressure. Thus, ratio of the total pressure to the original pressure is- a)2:1
- b)31:27
- c)27 : 37
- d)
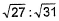
Correct answer is option 'B'. Can you explain this answer?
Ammonium carbamate dissociates as,
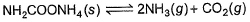
In a closed vessel containing ammonium carbamate in equilibrium with its vapour, ammonia is added such that partial pressure of NH3 now equals the original total pressure. Thus, ratio of the total pressure to the original pressure is
In a closed vessel containing ammonium carbamate in equilibrium with its vapour, ammonia is added such that partial pressure of NH3 now equals the original total pressure. Thus, ratio of the total pressure to the original pressure is
a)
2:1
b)
31:27
c)
27 : 37
d)

|
Imk Pathsala answered |
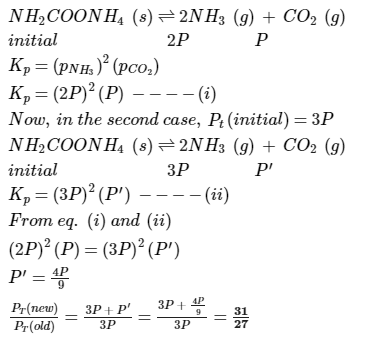
Which of the following will produce a buffer solution when mixed in equal volumes ?- a)0.1 mol dm–3 NH4OH and 0.1 mol dm–3 HCl
- b)0.05 mol dm–3 NH4OH and 0.1 mol dm–3 HCl
- c)0.1 mol dm–3 NH4OH and 0.05 mol dm–3 HCl
- d)0.1 mol dm–3 CH4COONa and 0.1 mol dm–3 NaOH
Correct answer is option 'C'. Can you explain this answer?
Which of the following will produce a buffer solution when mixed in equal volumes ?
a)
0.1 mol dm–3 NH4OH and 0.1 mol dm–3 HCl
b)
0.05 mol dm–3 NH4OH and 0.1 mol dm–3 HCl
c)
0.1 mol dm–3 NH4OH and 0.05 mol dm–3 HCl
d)
0.1 mol dm–3 CH4COONa and 0.1 mol dm–3 NaOH
|
|
Rahul Bansal answered |
In (c), all HCl will be neutralized and NH4Cl will be formed. Also some NH4OH will be left unneutralized. Thus, the final solution will contain NH4OH and NH4Cl and hence will form a buffer.
Can you explain the answer of this question below:Acidic strength is in order
- A:
H2O < CH3COOH < HF < NH3
- B:
NH3 < H2O < CH3COOH < HF
- C:
HF < CH3COOH < H2O < NH3
- D:
H2O < NH3 < HF < CH3COOH
The answer is b.
Acidic strength is in order
H2O < CH3COOH < HF < NH3
NH3 < H2O < CH3COOH < HF
HF < CH3COOH < H2O < NH3
H2O < NH3 < HF < CH3COOH
|
|
Preeti Khanna answered |
When we move from left to right, electronegativity of central atom increases. Due to this nature, central atom has more tendency to break its bond with H and acquire negative charge. So, while moving from left to right, acidic strength increase.
Consider the reaction equilibrium 2SO2(g)+O2(g)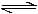 2 SO3 (g); ΔHº = -198 kJOn the basis of Le Chatelier's principle, the condition favourable for the forward reaction is -[AIEEE-2003]
2 SO3 (g); ΔHº = -198 kJOn the basis of Le Chatelier's principle, the condition favourable for the forward reaction is -[AIEEE-2003]- a)Lowering the temperature and increasing the pressure
- b)Any value of temperature and pressure
- c)Lowering of temperature as well as pressure
- d)Increasing temperature as well as pressure
Correct answer is option 'A'. Can you explain this answer?
Consider the reaction equilibrium 2SO2(g)+O2(g)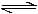 2 SO3 (g); ΔHº = -198 kJ
2 SO3 (g); ΔHº = -198 kJ
On the basis of Le Chatelier's principle, the condition favourable for the forward reaction is -
[AIEEE-2003]
a)
Lowering the temperature and increasing the pressure
b)
Any value of temperature and pressure
c)
Lowering of temperature as well as pressure
d)
Increasing temperature as well as pressure
|
|
Riya Banerjee answered |
The correct answer is option A
ACCORDING TO LE CHATELIER'S PRINCIPLE
TEMPERATURE
if we increase temperature equilibrium will shift that direction which proceeds an endothermic reaction but the reaction given in question is exothermic so we have to decrease temperature to proceed forward.
PRESSURE
at equilibrium if we increase pressure equilibrium will shift that direction which has less number of moles but in given reaction products have less no. of moles than reactant that's why we increase pressure.
ACCORDING TO LE CHATELIER'S PRINCIPLE
TEMPERATURE
if we increase temperature equilibrium will shift that direction which proceeds an endothermic reaction but the reaction given in question is exothermic so we have to decrease temperature to proceed forward.
PRESSURE
at equilibrium if we increase pressure equilibrium will shift that direction which has less number of moles but in given reaction products have less no. of moles than reactant that's why we increase pressure.
Passage IIA 15 L flask at 300 K contains 64.4 g of a mixture of NO2 and N2O4 in equilibrium. Given,
 Q. Kc for the above equilibrium is
Q. Kc for the above equilibrium is - a)164.28
- b) 6.087x 10-3
- c)0.2708
- d)3.693
Correct answer is option 'A'. Can you explain this answer?
Passage II
A 15 L flask at 300 K contains 64.4 g of a mixture of NO2 and N2O4 in equilibrium. Given,
Q. Kc for the above equilibrium is
a)
164.28
b)
6.087x 10-3
c)
0.2708
d)
3.693
|
|
Pooja Shah answered |
Kp = Kc(RT)∆n
Kp = 6.67 ,
∆n = moles of products - moles of reactants = 1-2 = -1
R = 0.0821 L atm mol-¹K-¹
T = 300K
∆n = moles of products - moles of reactants = 1-2 = -1
R = 0.0821 L atm mol-¹K-¹
T = 300K
Substitute these values in the formula,
=> Kc = 6.67×0.0821×300
Kc = 164.28.
=> Kc = 6.67×0.0821×300
Kc = 164.28.
The equilibrium constants Kp1 and Kp2 for the reactions X 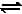 2Y and Z
2Y and Z 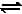 P + Q, respectively are in the ratio of 1 : 9. If the degree of dissociation of X and Z be equal then the ratio of total pressures at these equilibria is - [AIEEE 2008]
P + Q, respectively are in the ratio of 1 : 9. If the degree of dissociation of X and Z be equal then the ratio of total pressures at these equilibria is - [AIEEE 2008] - a)1 : 1
- b)1 : 3
- c)1 : 9
- d)1 :36
Correct answer is option 'D'. Can you explain this answer?
The equilibrium constants Kp1 and Kp2 for the reactions X 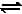 2Y and Z
2Y and Z 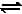 P + Q, respectively are in the ratio of 1 : 9. If the degree of dissociation of X and Z be equal then the ratio of total pressures at these equilibria is - [AIEEE 2008]
P + Q, respectively are in the ratio of 1 : 9. If the degree of dissociation of X and Z be equal then the ratio of total pressures at these equilibria is - [AIEEE 2008]
a)
1 : 1
b)
1 : 3
c)
1 : 9
d)
1 :36
|
|
Mira Sharma answered |
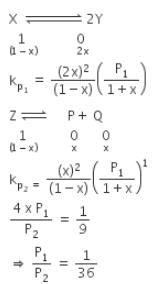
The equilibrium constant (Kc) for the reaction N2(g)+O2(g) → 2NO(g) at temperature T is4×10-4. The value of Kc for the reaction,
NO(g)→ ½ N2(g) + ½O2(g) at the same temperature is: [AIEEE 2012]- a)4x10-4
- b)50.0
- c)0.02
- d)2.5x102
Correct answer is option 'B'. Can you explain this answer?
The equilibrium constant (Kc) for the reaction N2(g)+O2(g) → 2NO(g) at temperature T is
4×10-4. The value of Kc for the reaction,
NO(g)→ ½ N2(g) + ½O2(g) at the same temperature is:
NO(g)→ ½ N2(g) + ½O2(g) at the same temperature is:
[AIEEE 2012]
a)
4x10-4
b)
50.0
c)
0.02
d)
2.5x102
|
|
Gaurav Kumar answered |
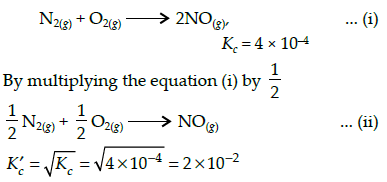
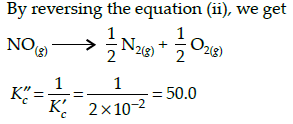
Chapter doubts & questions for Equilibrium - Physics - 4 Months Preparation for NEET 2025 is part of NEET exam preparation. The chapters have been prepared according to the NEET exam syllabus. The Chapter doubts & questions, notes, tests & MCQs are made for NEET 2025 Exam. Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests here.
Chapter doubts & questions of Equilibrium - Physics - 4 Months Preparation for NEET in English & Hindi are available as part of NEET exam.
Download more important topics, notes, lectures and mock test series for NEET Exam by signing up for free.

Contact Support
Our team is online on weekdays between 10 AM - 7 PM
Typical reply within 3 hours
|
Free Exam Preparation
at your Fingertips!
Access Free Study Material - Test Series, Structured Courses, Free Videos & Study Notes and Prepare for Your Exam With Ease

 Join the 10M+ students on EduRev
Join the 10M+ students on EduRev
|

|
Create your account for free
OR
Forgot Password
OR
Signup to see your scores
go up within 7 days!
Access 1000+ FREE Docs, Videos and Tests
Takes less than 10 seconds to signup