NEET Exam > NEET Questions > What does the negative electronic energy (En)...
Start Learning for Free
What does the negative electronic energy (En) for hydrogen atom mean?
Verified Answer
What does the negative electronic energy (En) for hydrogen atom mean?
The energy of the electron in a hydrogen atom has a negative sign for all possible orbits
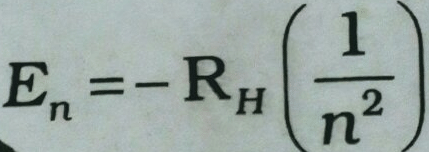
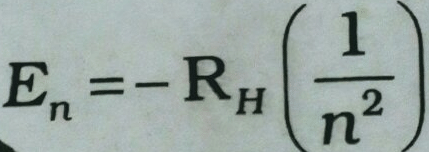
This negative sign means that the energy of the electron in the atom is lower than energy of a free electron at rest. A free electron at rest is an electron that is infinitely far away from the nucleus and is assigned the energy value of zero. Mathematically, this corresponds to setting n equal to infinity in the equation so that E infinity= 0. As the electron gets closer to the nucleus (as n decreases), E n becomes larger in absolute value and more and more negative. The most negative energy value is given by n=1 which corresponds to the most stable orbital. And We call this the Ground State .
 This question is part of UPSC exam. View all NEET courses
This question is part of UPSC exam. View all NEET courses
Most Upvoted Answer
What does the negative electronic energy (En) for hydrogen atom mean?
What does the negative electronic energy (En) for hydrogen atom mean?
The negative electronic energy (En) for a hydrogen atom refers to the amount of energy required to remove an electron from the hydrogen atom and place it at a distance infinitely far away from the atom. It is a measure of the stability of the atom and is directly related to the behavior of the electron within the atom.
Understanding the concept of electronic energy
- The electronic energy of an atom arises due to the interaction between the positively charged nucleus and the negatively charged electron(s).
- In the case of a hydrogen atom, which consists of a single electron orbiting a single proton, the electronic energy is determined by the electrostatic attraction between the electron and the proton.
The significance of negative electronic energy
- The negative sign of the electronic energy indicates that energy is released when an electron is bound to the hydrogen atom. This implies that the electron is in a lower energy state when it is in the atom compared to when it is infinitely far away.
- The negative electronic energy is a result of the attractive force between the electron and the proton, which allows the electron to be confined to a specific energy level within the atom.
- This negative energy is responsible for holding the electron in its orbit around the nucleus, preventing it from escaping and causing the atom to be stable.
Quantization of energy levels
- The electronic energy of the hydrogen atom is quantized, meaning it can only exist in certain discrete energy levels.
- Each energy level is associated with a specific value of negative electronic energy, denoted as En, where n is an integer representing the energy level.
- The energy levels are numbered as n=1, n=2, n=3, and so on, with n=1 being the ground state and higher values of n corresponding to higher energy levels.
Conclusion
In conclusion, the negative electronic energy for a hydrogen atom signifies the stability of the atom and the energy released when the electron is bound to the nucleus. The negative sign indicates that the electron is in a lower energy state within the atom compared to when it is infinitely far away. The quantization of energy levels in the hydrogen atom further demonstrates the discrete nature of electronic energy and the specific values associated with each energy level.
The negative electronic energy (En) for a hydrogen atom refers to the amount of energy required to remove an electron from the hydrogen atom and place it at a distance infinitely far away from the atom. It is a measure of the stability of the atom and is directly related to the behavior of the electron within the atom.
Understanding the concept of electronic energy
- The electronic energy of an atom arises due to the interaction between the positively charged nucleus and the negatively charged electron(s).
- In the case of a hydrogen atom, which consists of a single electron orbiting a single proton, the electronic energy is determined by the electrostatic attraction between the electron and the proton.
The significance of negative electronic energy
- The negative sign of the electronic energy indicates that energy is released when an electron is bound to the hydrogen atom. This implies that the electron is in a lower energy state when it is in the atom compared to when it is infinitely far away.
- The negative electronic energy is a result of the attractive force between the electron and the proton, which allows the electron to be confined to a specific energy level within the atom.
- This negative energy is responsible for holding the electron in its orbit around the nucleus, preventing it from escaping and causing the atom to be stable.
Quantization of energy levels
- The electronic energy of the hydrogen atom is quantized, meaning it can only exist in certain discrete energy levels.
- Each energy level is associated with a specific value of negative electronic energy, denoted as En, where n is an integer representing the energy level.
- The energy levels are numbered as n=1, n=2, n=3, and so on, with n=1 being the ground state and higher values of n corresponding to higher energy levels.
Conclusion
In conclusion, the negative electronic energy for a hydrogen atom signifies the stability of the atom and the energy released when the electron is bound to the nucleus. The negative sign indicates that the electron is in a lower energy state within the atom compared to when it is infinitely far away. The quantization of energy levels in the hydrogen atom further demonstrates the discrete nature of electronic energy and the specific values associated with each energy level.
Attention NEET Students!
To make sure you are not studying endlessly, EduRev has designed NEET study material, with Structured Courses, Videos, & Test Series. Plus get personalized analysis, doubt solving and improvement plans to achieve a great score in NEET.

|
Explore Courses for NEET exam
|

|
Similar NEET Doubts
What does the negative electronic energy (En) for hydrogen atom mean?
Question Description
What does the negative electronic energy (En) for hydrogen atom mean? for NEET 2024 is part of NEET preparation. The Question and answers have been prepared according to the NEET exam syllabus. Information about What does the negative electronic energy (En) for hydrogen atom mean? covers all topics & solutions for NEET 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for What does the negative electronic energy (En) for hydrogen atom mean? .
What does the negative electronic energy (En) for hydrogen atom mean? for NEET 2024 is part of NEET preparation. The Question and answers have been prepared according to the NEET exam syllabus. Information about What does the negative electronic energy (En) for hydrogen atom mean? covers all topics & solutions for NEET 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for What does the negative electronic energy (En) for hydrogen atom mean? .
Solutions for What does the negative electronic energy (En) for hydrogen atom mean? in English & in Hindi are available as part of our courses for NEET.
Download more important topics, notes, lectures and mock test series for NEET Exam by signing up for free.
Here you can find the meaning of What does the negative electronic energy (En) for hydrogen atom mean? defined & explained in the simplest way possible. Besides giving the explanation of
What does the negative electronic energy (En) for hydrogen atom mean? , a detailed solution for What does the negative electronic energy (En) for hydrogen atom mean? has been provided alongside types of What does the negative electronic energy (En) for hydrogen atom mean? theory, EduRev gives you an
ample number of questions to practice What does the negative electronic energy (En) for hydrogen atom mean? tests, examples and also practice NEET tests.

|
Explore Courses for NEET exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.

























