NEET Exam > NEET Questions > Topic : Isomerism? (Question attached in answ...
Start Learning for Free
Topic : Isomerism? (Question attached in answer section) How to solve? Correct ans (C)?
Most Upvoted Answer
Topic : Isomerism? (Question attached in answer section) How to solve?...
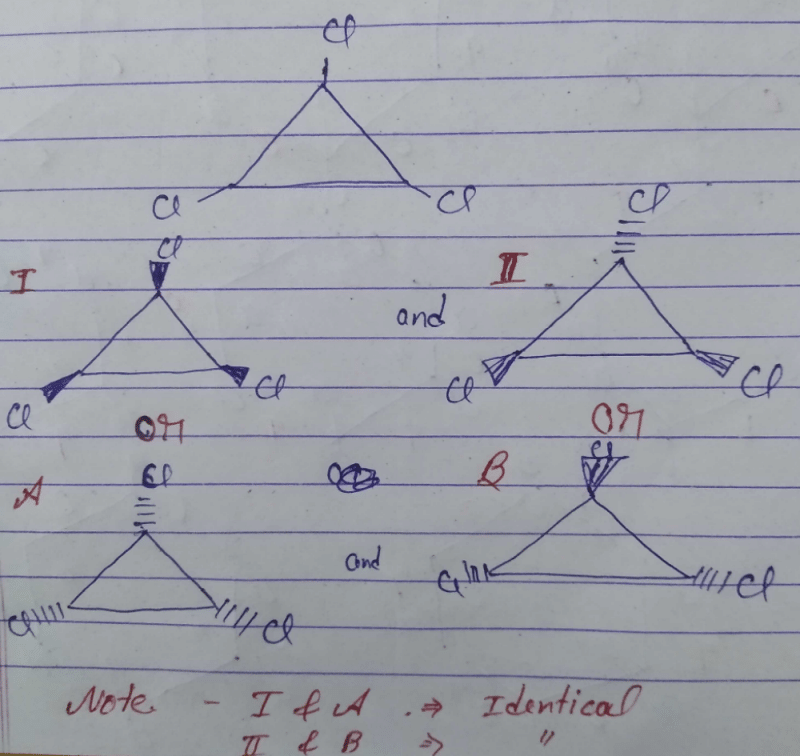
Community Answer
Topic : Isomerism? (Question attached in answer section) How to solve?...
Understanding Isomerism
Isomerism is a phenomenon where two or more compounds have the same molecular formula but different structural arrangements or spatial orientations. This can significantly affect their chemical properties and reactions.
Types of Isomerism
- Structural Isomerism: Different connectivity of atoms.
- Chain Isomerism: Variations in the carbon chain (e.g., straight vs. branched).
- Position Isomerism: Variations in the position of functional groups.
- Functional Group Isomerism: Different functional groups (e.g., alcohol vs. ether).
- Stereoisomerism: Same connectivity but different spatial arrangement.
- Geometric Isomerism (cis-trans): Different arrangements around a double bond or ring structure.
- Optical Isomerism: Non-superimposable mirror images, often involving chiral centers.
Why Isomerism Matters
- Chemical Properties: Isomers can exhibit vastly different physical and chemical properties. For instance, structural isomers may have different boiling points, solubilities, or reactivities.
- Biological Significance: Many biological molecules are isomers; their functions in biological systems depend on their specific structures.
Identifying Isomerism in a Given Question
To solve a question regarding isomerism, follow these steps:
1. Determine the Molecular Formula: Confirm that you are dealing with compounds that share the same molecular formula.
2. Analyze the Structure: Draw or visualize the structural representations to identify potential isomers.
3. Classify the Isomers: Determine whether they are structural or stereoisomers based on the arrangements of atoms.
4. Consider Properties: Think about how the differences in structure affect the properties and reactions of the isomers.
By following these guidelines, you can effectively analyze isomerism in various compounds, leading to a better understanding of their chemical behavior.
Isomerism is a phenomenon where two or more compounds have the same molecular formula but different structural arrangements or spatial orientations. This can significantly affect their chemical properties and reactions.
Types of Isomerism
- Structural Isomerism: Different connectivity of atoms.
- Chain Isomerism: Variations in the carbon chain (e.g., straight vs. branched).
- Position Isomerism: Variations in the position of functional groups.
- Functional Group Isomerism: Different functional groups (e.g., alcohol vs. ether).
- Stereoisomerism: Same connectivity but different spatial arrangement.
- Geometric Isomerism (cis-trans): Different arrangements around a double bond or ring structure.
- Optical Isomerism: Non-superimposable mirror images, often involving chiral centers.
Why Isomerism Matters
- Chemical Properties: Isomers can exhibit vastly different physical and chemical properties. For instance, structural isomers may have different boiling points, solubilities, or reactivities.
- Biological Significance: Many biological molecules are isomers; their functions in biological systems depend on their specific structures.
Identifying Isomerism in a Given Question
To solve a question regarding isomerism, follow these steps:
1. Determine the Molecular Formula: Confirm that you are dealing with compounds that share the same molecular formula.
2. Analyze the Structure: Draw or visualize the structural representations to identify potential isomers.
3. Classify the Isomers: Determine whether they are structural or stereoisomers based on the arrangements of atoms.
4. Consider Properties: Think about how the differences in structure affect the properties and reactions of the isomers.
By following these guidelines, you can effectively analyze isomerism in various compounds, leading to a better understanding of their chemical behavior.
Attention NEET Students!
To make sure you are not studying endlessly, EduRev has designed NEET study material, with Structured Courses, Videos, & Test Series. Plus get personalized analysis, doubt solving and improvement plans to achieve a great score in NEET.

|
Explore Courses for NEET exam
|

|
Similar NEET Doubts
Topic : Isomerism? (Question attached in answer section) How to solve? Correct ans (C)?
Question Description
Topic : Isomerism? (Question attached in answer section) How to solve? Correct ans (C)? for NEET 2024 is part of NEET preparation. The Question and answers have been prepared according to the NEET exam syllabus. Information about Topic : Isomerism? (Question attached in answer section) How to solve? Correct ans (C)? covers all topics & solutions for NEET 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Topic : Isomerism? (Question attached in answer section) How to solve? Correct ans (C)?.
Topic : Isomerism? (Question attached in answer section) How to solve? Correct ans (C)? for NEET 2024 is part of NEET preparation. The Question and answers have been prepared according to the NEET exam syllabus. Information about Topic : Isomerism? (Question attached in answer section) How to solve? Correct ans (C)? covers all topics & solutions for NEET 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Topic : Isomerism? (Question attached in answer section) How to solve? Correct ans (C)?.
Solutions for Topic : Isomerism? (Question attached in answer section) How to solve? Correct ans (C)? in English & in Hindi are available as part of our courses for NEET.
Download more important topics, notes, lectures and mock test series for NEET Exam by signing up for free.
Here you can find the meaning of Topic : Isomerism? (Question attached in answer section) How to solve? Correct ans (C)? defined & explained in the simplest way possible. Besides giving the explanation of
Topic : Isomerism? (Question attached in answer section) How to solve? Correct ans (C)?, a detailed solution for Topic : Isomerism? (Question attached in answer section) How to solve? Correct ans (C)? has been provided alongside types of Topic : Isomerism? (Question attached in answer section) How to solve? Correct ans (C)? theory, EduRev gives you an
ample number of questions to practice Topic : Isomerism? (Question attached in answer section) How to solve? Correct ans (C)? tests, examples and also practice NEET tests.

|
Explore Courses for NEET exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.

























