NEET Exam > NEET Questions > In the following solutions the concentration ...
Start Learning for Free
In the following solutions the concentration of different acids are given which mixture of the highest ph?
Most Upvoted Answer
In the following solutions the concentration of different acids are gi...
**Mixture of Highest pH**
To determine the mixture of the highest pH among the given solutions with different acid concentrations, we need to understand how acids and bases affect the pH of a solution. pH is a measure of the acidity or alkalinity of a solution, ranging from 0 to 14. A pH value below 7 indicates acidity, while a pH value above 7 indicates alkalinity. The higher the pH value, the more alkaline the solution is.
Now, let's analyze the given concentrations of different acids in the solutions and determine which mixture would have the highest pH.
**Solution 1: Hydrochloric Acid (HCl) - 0.1 M**
Hydrochloric acid is a strong acid that completely dissociates in water, producing a high concentration of hydrogen ions (H+). As a result, the solution will have a low pH, indicating high acidity.
**Solution 2: Acetic Acid (CH3COOH) - 0.01 M**
Acetic acid is a weak acid that only partially dissociates in water, resulting in a lower concentration of hydrogen ions compared to hydrochloric acid. However, it still contributes to the acidity of the solution, albeit to a lesser extent than a strong acid like HCl.
**Solution 3: Sulfuric Acid (H2SO4) - 1 M**
Sulfuric acid is another strong acid that completely dissociates in water, similar to hydrochloric acid. Hence, the solution will have a low pH, indicating high acidity.
**Solution 4: Nitric Acid (HNO3) - 0.001 M**
Nitric acid is also a strong acid that fully ionizes in water, leading to a high concentration of hydrogen ions and a low pH.
**Solution 5: Phosphoric Acid (H3PO4) - 0.1 M**
Phosphoric acid is a weak acid that partially ionizes in water. It has three acidic hydrogens, so it can release multiple hydrogen ions, contributing to the acidity of the solution. However, the concentration of hydrogen ions will be lower compared to strong acids like HCl or H2SO4.
**Solution 6: Citric Acid (C6H8O7) - 0.0001 M**
Citric acid is a weak acid commonly found in citrus fruits. It also partially ionizes in water, resulting in a lower concentration of hydrogen ions compared to strong acids.
Based on the analysis of the acid concentrations, we can conclude that **Solution 6: Citric Acid (C6H8O7) - 0.0001 M** would likely have the highest pH among the given mixtures. Since citric acid is a weak acid with the lowest concentration, it will produce a relatively lower concentration of hydrogen ions compared to the other strong acids. Consequently, the pH of the solution will be higher, indicating a more alkaline nature. However, it is important to note that the exact pH values cannot be determined without additional information, such as the pKa values of the acids and their respective dissociation constants.
To determine the mixture of the highest pH among the given solutions with different acid concentrations, we need to understand how acids and bases affect the pH of a solution. pH is a measure of the acidity or alkalinity of a solution, ranging from 0 to 14. A pH value below 7 indicates acidity, while a pH value above 7 indicates alkalinity. The higher the pH value, the more alkaline the solution is.
Now, let's analyze the given concentrations of different acids in the solutions and determine which mixture would have the highest pH.
**Solution 1: Hydrochloric Acid (HCl) - 0.1 M**
Hydrochloric acid is a strong acid that completely dissociates in water, producing a high concentration of hydrogen ions (H+). As a result, the solution will have a low pH, indicating high acidity.
**Solution 2: Acetic Acid (CH3COOH) - 0.01 M**
Acetic acid is a weak acid that only partially dissociates in water, resulting in a lower concentration of hydrogen ions compared to hydrochloric acid. However, it still contributes to the acidity of the solution, albeit to a lesser extent than a strong acid like HCl.
**Solution 3: Sulfuric Acid (H2SO4) - 1 M**
Sulfuric acid is another strong acid that completely dissociates in water, similar to hydrochloric acid. Hence, the solution will have a low pH, indicating high acidity.
**Solution 4: Nitric Acid (HNO3) - 0.001 M**
Nitric acid is also a strong acid that fully ionizes in water, leading to a high concentration of hydrogen ions and a low pH.
**Solution 5: Phosphoric Acid (H3PO4) - 0.1 M**
Phosphoric acid is a weak acid that partially ionizes in water. It has three acidic hydrogens, so it can release multiple hydrogen ions, contributing to the acidity of the solution. However, the concentration of hydrogen ions will be lower compared to strong acids like HCl or H2SO4.
**Solution 6: Citric Acid (C6H8O7) - 0.0001 M**
Citric acid is a weak acid commonly found in citrus fruits. It also partially ionizes in water, resulting in a lower concentration of hydrogen ions compared to strong acids.
Based on the analysis of the acid concentrations, we can conclude that **Solution 6: Citric Acid (C6H8O7) - 0.0001 M** would likely have the highest pH among the given mixtures. Since citric acid is a weak acid with the lowest concentration, it will produce a relatively lower concentration of hydrogen ions compared to the other strong acids. Consequently, the pH of the solution will be higher, indicating a more alkaline nature. However, it is important to note that the exact pH values cannot be determined without additional information, such as the pKa values of the acids and their respective dissociation constants.
Community Answer
In the following solutions the concentration of different acids are gi...
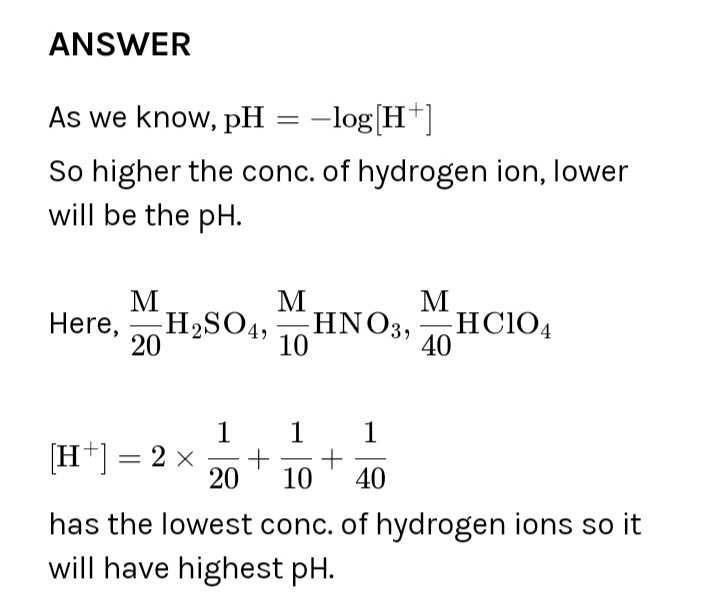
Attention NEET Students!
To make sure you are not studying endlessly, EduRev has designed NEET study material, with Structured Courses, Videos, & Test Series. Plus get personalized analysis, doubt solving and improvement plans to achieve a great score in NEET.

|
Explore Courses for NEET exam
|

|
Similar NEET Doubts
In the following solutions the concentration of different acids are given which mixture of the highest ph?
Question Description
In the following solutions the concentration of different acids are given which mixture of the highest ph? for NEET 2024 is part of NEET preparation. The Question and answers have been prepared according to the NEET exam syllabus. Information about In the following solutions the concentration of different acids are given which mixture of the highest ph? covers all topics & solutions for NEET 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for In the following solutions the concentration of different acids are given which mixture of the highest ph?.
In the following solutions the concentration of different acids are given which mixture of the highest ph? for NEET 2024 is part of NEET preparation. The Question and answers have been prepared according to the NEET exam syllabus. Information about In the following solutions the concentration of different acids are given which mixture of the highest ph? covers all topics & solutions for NEET 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for In the following solutions the concentration of different acids are given which mixture of the highest ph?.
Solutions for In the following solutions the concentration of different acids are given which mixture of the highest ph? in English & in Hindi are available as part of our courses for NEET.
Download more important topics, notes, lectures and mock test series for NEET Exam by signing up for free.
Here you can find the meaning of In the following solutions the concentration of different acids are given which mixture of the highest ph? defined & explained in the simplest way possible. Besides giving the explanation of
In the following solutions the concentration of different acids are given which mixture of the highest ph?, a detailed solution for In the following solutions the concentration of different acids are given which mixture of the highest ph? has been provided alongside types of In the following solutions the concentration of different acids are given which mixture of the highest ph? theory, EduRev gives you an
ample number of questions to practice In the following solutions the concentration of different acids are given which mixture of the highest ph? tests, examples and also practice NEET tests.

|
Explore Courses for NEET exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.

























