NEET Exam > NEET Questions > Select the incorrect statement about the XeF6...
Start Learning for Free
Select the incorrect statement about the XeF6.
- a)It has capped octahedron or distorted octahedral structure with one lone pair.
- b)In various non-aqueous solvents it forms a tetramer Xe4F24.
- c)Solid Xenon hexafluoride is polymorphic.
- d)None of these
Correct answer is option 'D'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
Select the incorrect statement about the XeF6.a)It has capped octahedr...
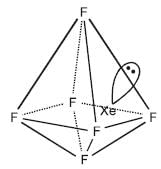 Capped octahedron (or distorted octahedron)
Capped octahedron (or distorted octahedron) In tetramer, four square pyramidal XeF5+ ions are joined to two similar ions by means of two bridging F- ions. The Xe-F distances are 1.84 A on the square pyramidal units and 2.23 A and 2.60 A in the bridging groups. The solid has various crystalline forms, of which three are tetrameric and a fourth has both hexamers and tetramers.
Most Upvoted Answer
Select the incorrect statement about the XeF6.a)It has capped octahedr...
Incorrect Statement: It has capped octahedron or distorted octahedral structure with one lone pair.
Explanation:
Xenon hexafluoride (XeF6) is an inorganic compound consisting of xenon and fluorine atoms. It is a powerful fluorinating agent and is commonly used in chemical reactions to introduce fluorine atoms into organic molecules.
XeF6 does not have a capped octahedron or distorted octahedral structure with one lone pair. Instead, it has an octahedral structure with no lone pairs. The central xenon atom is surrounded by six fluorine atoms, forming a regular octahedron. Each fluorine atom is bonded to the xenon atom through a single covalent bond, resulting in a total of six bonds.
The correct statement regarding the structure of XeF6 is that it has an octahedral structure with no lone pairs. This is important to note because the absence of lone pairs affects the reactivity and chemical behavior of XeF6.
Other Statements:
a) In various non-aqueous solvents, it forms a tetramer Xe4F24.
This statement is correct. XeF6 can undergo self-association in non-aqueous solvents, leading to the formation of a tetrameric species, Xe4F24. In this tetramer, four XeF6 molecules are connected through bridging fluorine atoms, resulting in a cluster with a total of 24 fluorine atoms.
b) Solid Xenon hexafluoride is polymorphic.
This statement is correct. XeF6 exhibits polymorphism, which means it can exist in different crystal structures. At low temperatures, XeF6 crystallizes in a monoclinic structure, while at higher temperatures, it transforms into a cubic structure. This ability to exist in multiple crystal structures is a characteristic feature of XeF6.
c) None of these.
This statement is incorrect as explained above.
In summary, the incorrect statement is that XeF6 has a capped octahedron or distorted octahedral structure with one lone pair. The correct statement is that XeF6 has an octahedral structure with no lone pairs.
Explanation:
Xenon hexafluoride (XeF6) is an inorganic compound consisting of xenon and fluorine atoms. It is a powerful fluorinating agent and is commonly used in chemical reactions to introduce fluorine atoms into organic molecules.
XeF6 does not have a capped octahedron or distorted octahedral structure with one lone pair. Instead, it has an octahedral structure with no lone pairs. The central xenon atom is surrounded by six fluorine atoms, forming a regular octahedron. Each fluorine atom is bonded to the xenon atom through a single covalent bond, resulting in a total of six bonds.
The correct statement regarding the structure of XeF6 is that it has an octahedral structure with no lone pairs. This is important to note because the absence of lone pairs affects the reactivity and chemical behavior of XeF6.
Other Statements:
a) In various non-aqueous solvents, it forms a tetramer Xe4F24.
This statement is correct. XeF6 can undergo self-association in non-aqueous solvents, leading to the formation of a tetrameric species, Xe4F24. In this tetramer, four XeF6 molecules are connected through bridging fluorine atoms, resulting in a cluster with a total of 24 fluorine atoms.
b) Solid Xenon hexafluoride is polymorphic.
This statement is correct. XeF6 exhibits polymorphism, which means it can exist in different crystal structures. At low temperatures, XeF6 crystallizes in a monoclinic structure, while at higher temperatures, it transforms into a cubic structure. This ability to exist in multiple crystal structures is a characteristic feature of XeF6.
c) None of these.
This statement is incorrect as explained above.
In summary, the incorrect statement is that XeF6 has a capped octahedron or distorted octahedral structure with one lone pair. The correct statement is that XeF6 has an octahedral structure with no lone pairs.
Attention NEET Students!
To make sure you are not studying endlessly, EduRev has designed NEET study material, with Structured Courses, Videos, & Test Series. Plus get personalized analysis, doubt solving and improvement plans to achieve a great score in NEET.

|
Explore Courses for NEET exam
|

|
Similar NEET Doubts
Select the incorrect statement about the XeF6.a)It has capped octahedron or distorted octahedral structure with one lone pair.b)In various non-aqueous solvents it forms a tetramer Xe4F24.c)Solid Xenon hexafluoride is polymorphic.d)None of theseCorrect answer is option 'D'. Can you explain this answer?
Question Description
Select the incorrect statement about the XeF6.a)It has capped octahedron or distorted octahedral structure with one lone pair.b)In various non-aqueous solvents it forms a tetramer Xe4F24.c)Solid Xenon hexafluoride is polymorphic.d)None of theseCorrect answer is option 'D'. Can you explain this answer? for NEET 2024 is part of NEET preparation. The Question and answers have been prepared according to the NEET exam syllabus. Information about Select the incorrect statement about the XeF6.a)It has capped octahedron or distorted octahedral structure with one lone pair.b)In various non-aqueous solvents it forms a tetramer Xe4F24.c)Solid Xenon hexafluoride is polymorphic.d)None of theseCorrect answer is option 'D'. Can you explain this answer? covers all topics & solutions for NEET 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Select the incorrect statement about the XeF6.a)It has capped octahedron or distorted octahedral structure with one lone pair.b)In various non-aqueous solvents it forms a tetramer Xe4F24.c)Solid Xenon hexafluoride is polymorphic.d)None of theseCorrect answer is option 'D'. Can you explain this answer?.
Select the incorrect statement about the XeF6.a)It has capped octahedron or distorted octahedral structure with one lone pair.b)In various non-aqueous solvents it forms a tetramer Xe4F24.c)Solid Xenon hexafluoride is polymorphic.d)None of theseCorrect answer is option 'D'. Can you explain this answer? for NEET 2024 is part of NEET preparation. The Question and answers have been prepared according to the NEET exam syllabus. Information about Select the incorrect statement about the XeF6.a)It has capped octahedron or distorted octahedral structure with one lone pair.b)In various non-aqueous solvents it forms a tetramer Xe4F24.c)Solid Xenon hexafluoride is polymorphic.d)None of theseCorrect answer is option 'D'. Can you explain this answer? covers all topics & solutions for NEET 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Select the incorrect statement about the XeF6.a)It has capped octahedron or distorted octahedral structure with one lone pair.b)In various non-aqueous solvents it forms a tetramer Xe4F24.c)Solid Xenon hexafluoride is polymorphic.d)None of theseCorrect answer is option 'D'. Can you explain this answer?.
Solutions for Select the incorrect statement about the XeF6.a)It has capped octahedron or distorted octahedral structure with one lone pair.b)In various non-aqueous solvents it forms a tetramer Xe4F24.c)Solid Xenon hexafluoride is polymorphic.d)None of theseCorrect answer is option 'D'. Can you explain this answer? in English & in Hindi are available as part of our courses for NEET.
Download more important topics, notes, lectures and mock test series for NEET Exam by signing up for free.
Here you can find the meaning of Select the incorrect statement about the XeF6.a)It has capped octahedron or distorted octahedral structure with one lone pair.b)In various non-aqueous solvents it forms a tetramer Xe4F24.c)Solid Xenon hexafluoride is polymorphic.d)None of theseCorrect answer is option 'D'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Select the incorrect statement about the XeF6.a)It has capped octahedron or distorted octahedral structure with one lone pair.b)In various non-aqueous solvents it forms a tetramer Xe4F24.c)Solid Xenon hexafluoride is polymorphic.d)None of theseCorrect answer is option 'D'. Can you explain this answer?, a detailed solution for Select the incorrect statement about the XeF6.a)It has capped octahedron or distorted octahedral structure with one lone pair.b)In various non-aqueous solvents it forms a tetramer Xe4F24.c)Solid Xenon hexafluoride is polymorphic.d)None of theseCorrect answer is option 'D'. Can you explain this answer? has been provided alongside types of Select the incorrect statement about the XeF6.a)It has capped octahedron or distorted octahedral structure with one lone pair.b)In various non-aqueous solvents it forms a tetramer Xe4F24.c)Solid Xenon hexafluoride is polymorphic.d)None of theseCorrect answer is option 'D'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Select the incorrect statement about the XeF6.a)It has capped octahedron or distorted octahedral structure with one lone pair.b)In various non-aqueous solvents it forms a tetramer Xe4F24.c)Solid Xenon hexafluoride is polymorphic.d)None of theseCorrect answer is option 'D'. Can you explain this answer? tests, examples and also practice NEET tests.

|
Explore Courses for NEET exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
























