GATE Exam > GATE Questions > Which of the following are statement is/are c...
Start Learning for Free
Which of the following are statement is/are correct :-
- a)For all substances the specific volume in vapour phase is always larger than that in the liquid phase.
- b)The boiling point is raised by an increase in pressure.
- c)The melting point of substance, which expands on melting, increases with increase of pressure.
- d)The melting point of substance which contracts on melting, decreases with increases of pressure.
Correct answer is option 'A,B,C,D'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
Which of the following are statement is/are correct :-a)For all substa...
In case of boiling, V2 represents the specific volume of the vapour and V1 that of the liquid phase of the substance.
For all substance the specific volume in vapour phase is always larger than that in the liquid phase.
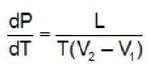
Hence, in the case of the boiling point of a liquid an increases in the pressure (dP > 0) will give a positive value of dT.
It means the boiling point is raised by an increase in pressure.
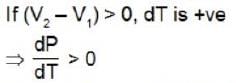
The melting point of substance, which expands on melting increases with increase of pressure.
(V2 - V1) < 0
Melting point of substance which contracts on melting, decreases with increases of pressure.
For all substance the specific volume in vapour phase is always larger than that in the liquid phase.
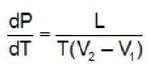
Hence, in the case of the boiling point of a liquid an increases in the pressure (dP > 0) will give a positive value of dT.
It means the boiling point is raised by an increase in pressure.
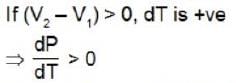
The melting point of substance, which expands on melting increases with increase of pressure.
(V2 - V1) < 0
Melting point of substance which contracts on melting, decreases with increases of pressure.
Most Upvoted Answer
Which of the following are statement is/are correct :-a)For all substa...
Statement A: Specific Volume in Vapor vs. Liquid Phase
The specific volume of a substance in the vapor phase is indeed always larger than that in the liquid phase.
- Reason: In the vapor phase, molecules are much farther apart compared to the liquid phase, where they are closely packed.
Statement B: Boiling Point and Pressure Relation
The boiling point of a substance increases with an increase in pressure.
- Reason: Higher pressure raises the boiling point because it requires more energy (higher temperature) for the vapor pressure of the liquid to equal the external pressure.
Statement C: Melting Point with Increased Pressure for Expanding Substances
For substances that expand upon melting, the melting point increases with an increase in pressure.
- Reason: When pressure is applied, it favors the denser phase. Since these substances expand when they melt, an increase in pressure raises the melting point to favor the solid state.
Statement D: Melting Point with Increased Pressure for Contracting Substances
For substances that contract upon melting, the melting point decreases with an increase in pressure.
- Reason: In this case, the solid phase is denser. Increased pressure favors the solid state, thus lowering the melting point.
Conclusion
All the statements A, B, C, and D are correct based on the principles of thermodynamics and the behavior of different phases of substances under varying conditions of pressure and temperature. Understanding these concepts is crucial for applications in physical chemistry and engineering disciplines.
The specific volume of a substance in the vapor phase is indeed always larger than that in the liquid phase.
- Reason: In the vapor phase, molecules are much farther apart compared to the liquid phase, where they are closely packed.
Statement B: Boiling Point and Pressure Relation
The boiling point of a substance increases with an increase in pressure.
- Reason: Higher pressure raises the boiling point because it requires more energy (higher temperature) for the vapor pressure of the liquid to equal the external pressure.
Statement C: Melting Point with Increased Pressure for Expanding Substances
For substances that expand upon melting, the melting point increases with an increase in pressure.
- Reason: When pressure is applied, it favors the denser phase. Since these substances expand when they melt, an increase in pressure raises the melting point to favor the solid state.
Statement D: Melting Point with Increased Pressure for Contracting Substances
For substances that contract upon melting, the melting point decreases with an increase in pressure.
- Reason: In this case, the solid phase is denser. Increased pressure favors the solid state, thus lowering the melting point.
Conclusion
All the statements A, B, C, and D are correct based on the principles of thermodynamics and the behavior of different phases of substances under varying conditions of pressure and temperature. Understanding these concepts is crucial for applications in physical chemistry and engineering disciplines.

|
Explore Courses for GATE exam
|

|
Which of the following are statement is/are correct :-a)For all substances the specific volume in vapour phase is always larger than that in the liquid phase.b)The boiling point is raised by an increase in pressure.c)The melting point of substance, which expands on melting, increases with increase of pressure.d)The melting point of substance which contracts on melting, decreases with increases of pressure.Correct answer is option 'A,B,C,D'. Can you explain this answer?
Question Description
Which of the following are statement is/are correct :-a)For all substances the specific volume in vapour phase is always larger than that in the liquid phase.b)The boiling point is raised by an increase in pressure.c)The melting point of substance, which expands on melting, increases with increase of pressure.d)The melting point of substance which contracts on melting, decreases with increases of pressure.Correct answer is option 'A,B,C,D'. Can you explain this answer? for GATE 2024 is part of GATE preparation. The Question and answers have been prepared according to the GATE exam syllabus. Information about Which of the following are statement is/are correct :-a)For all substances the specific volume in vapour phase is always larger than that in the liquid phase.b)The boiling point is raised by an increase in pressure.c)The melting point of substance, which expands on melting, increases with increase of pressure.d)The melting point of substance which contracts on melting, decreases with increases of pressure.Correct answer is option 'A,B,C,D'. Can you explain this answer? covers all topics & solutions for GATE 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Which of the following are statement is/are correct :-a)For all substances the specific volume in vapour phase is always larger than that in the liquid phase.b)The boiling point is raised by an increase in pressure.c)The melting point of substance, which expands on melting, increases with increase of pressure.d)The melting point of substance which contracts on melting, decreases with increases of pressure.Correct answer is option 'A,B,C,D'. Can you explain this answer?.
Which of the following are statement is/are correct :-a)For all substances the specific volume in vapour phase is always larger than that in the liquid phase.b)The boiling point is raised by an increase in pressure.c)The melting point of substance, which expands on melting, increases with increase of pressure.d)The melting point of substance which contracts on melting, decreases with increases of pressure.Correct answer is option 'A,B,C,D'. Can you explain this answer? for GATE 2024 is part of GATE preparation. The Question and answers have been prepared according to the GATE exam syllabus. Information about Which of the following are statement is/are correct :-a)For all substances the specific volume in vapour phase is always larger than that in the liquid phase.b)The boiling point is raised by an increase in pressure.c)The melting point of substance, which expands on melting, increases with increase of pressure.d)The melting point of substance which contracts on melting, decreases with increases of pressure.Correct answer is option 'A,B,C,D'. Can you explain this answer? covers all topics & solutions for GATE 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Which of the following are statement is/are correct :-a)For all substances the specific volume in vapour phase is always larger than that in the liquid phase.b)The boiling point is raised by an increase in pressure.c)The melting point of substance, which expands on melting, increases with increase of pressure.d)The melting point of substance which contracts on melting, decreases with increases of pressure.Correct answer is option 'A,B,C,D'. Can you explain this answer?.
Solutions for Which of the following are statement is/are correct :-a)For all substances the specific volume in vapour phase is always larger than that in the liquid phase.b)The boiling point is raised by an increase in pressure.c)The melting point of substance, which expands on melting, increases with increase of pressure.d)The melting point of substance which contracts on melting, decreases with increases of pressure.Correct answer is option 'A,B,C,D'. Can you explain this answer? in English & in Hindi are available as part of our courses for GATE.
Download more important topics, notes, lectures and mock test series for GATE Exam by signing up for free.
Here you can find the meaning of Which of the following are statement is/are correct :-a)For all substances the specific volume in vapour phase is always larger than that in the liquid phase.b)The boiling point is raised by an increase in pressure.c)The melting point of substance, which expands on melting, increases with increase of pressure.d)The melting point of substance which contracts on melting, decreases with increases of pressure.Correct answer is option 'A,B,C,D'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Which of the following are statement is/are correct :-a)For all substances the specific volume in vapour phase is always larger than that in the liquid phase.b)The boiling point is raised by an increase in pressure.c)The melting point of substance, which expands on melting, increases with increase of pressure.d)The melting point of substance which contracts on melting, decreases with increases of pressure.Correct answer is option 'A,B,C,D'. Can you explain this answer?, a detailed solution for Which of the following are statement is/are correct :-a)For all substances the specific volume in vapour phase is always larger than that in the liquid phase.b)The boiling point is raised by an increase in pressure.c)The melting point of substance, which expands on melting, increases with increase of pressure.d)The melting point of substance which contracts on melting, decreases with increases of pressure.Correct answer is option 'A,B,C,D'. Can you explain this answer? has been provided alongside types of Which of the following are statement is/are correct :-a)For all substances the specific volume in vapour phase is always larger than that in the liquid phase.b)The boiling point is raised by an increase in pressure.c)The melting point of substance, which expands on melting, increases with increase of pressure.d)The melting point of substance which contracts on melting, decreases with increases of pressure.Correct answer is option 'A,B,C,D'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Which of the following are statement is/are correct :-a)For all substances the specific volume in vapour phase is always larger than that in the liquid phase.b)The boiling point is raised by an increase in pressure.c)The melting point of substance, which expands on melting, increases with increase of pressure.d)The melting point of substance which contracts on melting, decreases with increases of pressure.Correct answer is option 'A,B,C,D'. Can you explain this answer? tests, examples and also practice GATE tests.

|
Explore Courses for GATE exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















