Class 10 Exam > Class 10 Questions > Difference between saponification and esterif...
Start Learning for Free
Difference between saponification and esterification ?
Most Upvoted Answer
Difference between saponification and esterification ?
Community Answer
Difference between saponification and esterification ?
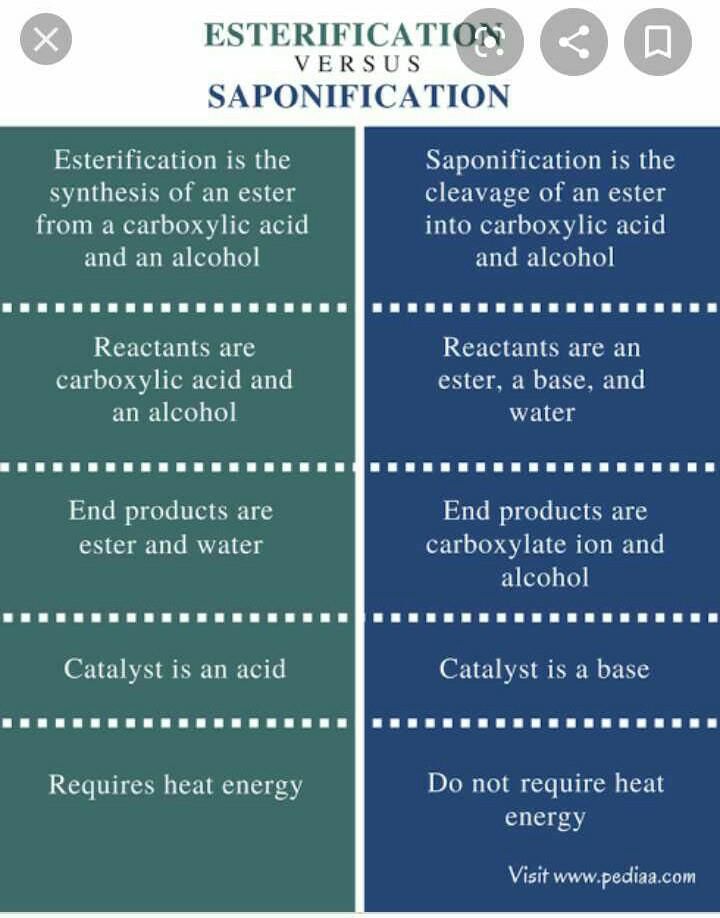
Saponification vs. Esterification
Saponification:
- Saponification is a chemical reaction that involves the conversion of a fat or oil into soap and alcohol by the action of heat, alkali, and water.
- It is a hydrolysis reaction in which ester bonds are broken down by the addition of water, resulting in the formation of soap (salts of fatty acids) and glycerol.
- Saponification is commonly used in the soap-making process, where fats or oils are mixed with a strong alkali such as sodium hydroxide or potassium hydroxide.
- The reaction produces soap molecules, which have a hydrophilic (water-attracting) head and a hydrophobic (water-repelling) tail, allowing them to act as surfactants.
- Saponification is a reversible reaction, and the products can be converted back into fats and oils by the addition of acid.
Esterification:
- Esterification is a chemical reaction that involves the formation of an ester from an alcohol and a carboxylic acid in the presence of an acid catalyst.
- It is a condensation reaction in which water is eliminated as a byproduct, and an ester bond is formed between the alcohol and carboxylic acid molecules.
- Esterification is commonly used in the synthesis of various esters, which are important compounds in the fragrance, flavor, and pharmaceutical industries.
- The reaction can be carried out under reflux conditions to drive the equilibrium towards the formation of the ester product.
- Esterification is a reversible reaction, and the ester product can be hydrolyzed back into the alcohol and carboxylic acid components by the addition of water and heat.
In conclusion, saponification and esterification are two important chemical reactions with distinct mechanisms and applications in various industries.
Attention Class 10 Students!
To make sure you are not studying endlessly, EduRev has designed Class 10 study material, with Structured Courses, Videos, & Test Series. Plus get personalized analysis, doubt solving and improvement plans to achieve a great score in Class 10.

|
Explore Courses for Class 10 exam
|

|
Similar Class 10 Doubts
Difference between saponification and esterification ?
Question Description
Difference between saponification and esterification ? for Class 10 2024 is part of Class 10 preparation. The Question and answers have been prepared according to the Class 10 exam syllabus. Information about Difference between saponification and esterification ? covers all topics & solutions for Class 10 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Difference between saponification and esterification ?.
Difference between saponification and esterification ? for Class 10 2024 is part of Class 10 preparation. The Question and answers have been prepared according to the Class 10 exam syllabus. Information about Difference between saponification and esterification ? covers all topics & solutions for Class 10 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Difference between saponification and esterification ?.
Solutions for Difference between saponification and esterification ? in English & in Hindi are available as part of our courses for Class 10.
Download more important topics, notes, lectures and mock test series for Class 10 Exam by signing up for free.
Here you can find the meaning of Difference between saponification and esterification ? defined & explained in the simplest way possible. Besides giving the explanation of
Difference between saponification and esterification ?, a detailed solution for Difference between saponification and esterification ? has been provided alongside types of Difference between saponification and esterification ? theory, EduRev gives you an
ample number of questions to practice Difference between saponification and esterification ? tests, examples and also practice Class 10 tests.

|
Explore Courses for Class 10 exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.

























