NEET Exam > NEET Questions > NEET PREPARATION SERIES (Formula sheet for So...
Start Learning for Free
NEET PREPARATION SERIES (Formula sheet for Solid State)?
Most Upvoted Answer
NEET PREPARATION SERIES (Formula sheet for Solid State)?
Community Answer
NEET PREPARATION SERIES (Formula sheet for Solid State)?
**Formula sheet for Solid State**
Solid State is an important topic in the NEET examination, and having a formula sheet handy can be extremely helpful for quick revision and problem-solving. In this article, we will provide you with a detailed formula sheet for Solid State, covering the key concepts and equations you need to know.
**1. Bravais Lattice**
- Bravais lattice: A lattice is an imaginary framework in which the points represent the constituent particles of a crystal.
- There are 14 possible Bravais lattices in three dimensions, categorized into seven crystal systems.
- Crystal systems: Cubic, Tetragonal, Orthorhombic, Rhombohedral, Monoclinic, Triclinic, Hexagonal.
**2. Unit Cell**
- Unit cell: The smallest repeating unit of a crystal lattice.
- Types of unit cells: Primitive, Body-centered (bcc), Face-centered (fcc), and Hexagonal.
**3. Density Calculation**
- Density (ρ) = (Z × M) / (a^3 × N_A), where Z is the number of atoms in the unit cell, M is the molar mass of the substance, a is the length of the edge of the unit cell, and N_A is Avogadro's number.
**4. Imperfections in Solids**
- Point defects: Vacancies, Interstitials, Substitutional, and Frenkel defects.
- Line defects: Edge dislocations and Screw dislocations.
- Surface defects: Steps, Kinks, and Terraces.
**5. Crystal Systems and Coordination Number**
- Cubic: Coordination number (CN) = 6
- Tetragonal: CN = 4 or 8
- Orthorhombic: CN = 4, 6, or 8
- Rhombohedral: CN = 3, 4, or 6
- Monoclinic: CN = 4, 6, or 8
- Triclinic: CN = 4, 6, or 8
- Hexagonal: CN = 6
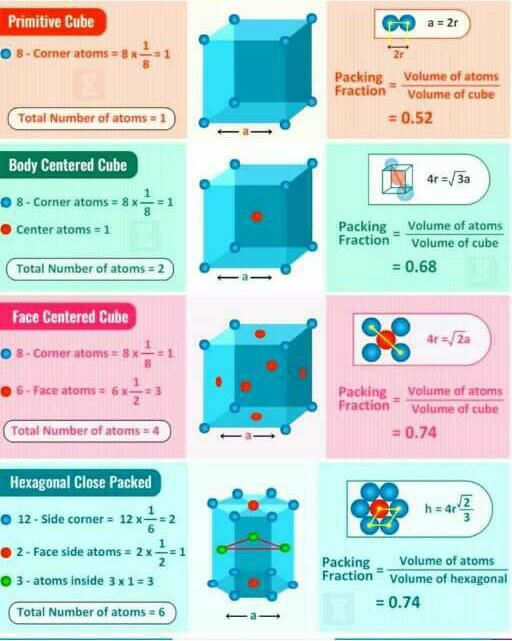
**6. Packing Efficiency**
- Packing efficiency = (Volume occupied by spheres) / (Total volume of unit cell)
- Cubic (primitive) packing efficiency = π / 6 ≈ 0.52
- Cubic (body-centered) packing efficiency = π / 3 ≈ 0.68
- Cubic (face-centered) packing efficiency = π / 2 ≈ 0.74
- Hexagonal packing efficiency = 0.74
**7. X-Ray Diffraction**
- Bragg's law: nλ = 2d sinθ, where n is the order of the diffraction, λ is the wavelength of X-rays, d is the distance between atomic planes, and θ is the angle of incidence.
**8. Electrical Conductivity**
- Conductors: High electrical conductivity due to the presence of a large number of free electrons.
- Insulators: Poor electrical conductivity due to a completely filled valence band and large energy gap.
- Semiconductors: Moderate electrical conductivity due to a partially filled valence band and a small energy gap.
These are some of the key formulas and concepts that you should include in your Solid State formula sheet for NEET preparation. Regular revision and practice using these formulas will enhance your understanding and problem
Solid State is an important topic in the NEET examination, and having a formula sheet handy can be extremely helpful for quick revision and problem-solving. In this article, we will provide you with a detailed formula sheet for Solid State, covering the key concepts and equations you need to know.
**1. Bravais Lattice**
- Bravais lattice: A lattice is an imaginary framework in which the points represent the constituent particles of a crystal.
- There are 14 possible Bravais lattices in three dimensions, categorized into seven crystal systems.
- Crystal systems: Cubic, Tetragonal, Orthorhombic, Rhombohedral, Monoclinic, Triclinic, Hexagonal.
**2. Unit Cell**
- Unit cell: The smallest repeating unit of a crystal lattice.
- Types of unit cells: Primitive, Body-centered (bcc), Face-centered (fcc), and Hexagonal.
**3. Density Calculation**
- Density (ρ) = (Z × M) / (a^3 × N_A), where Z is the number of atoms in the unit cell, M is the molar mass of the substance, a is the length of the edge of the unit cell, and N_A is Avogadro's number.
**4. Imperfections in Solids**
- Point defects: Vacancies, Interstitials, Substitutional, and Frenkel defects.
- Line defects: Edge dislocations and Screw dislocations.
- Surface defects: Steps, Kinks, and Terraces.
**5. Crystal Systems and Coordination Number**
- Cubic: Coordination number (CN) = 6
- Tetragonal: CN = 4 or 8
- Orthorhombic: CN = 4, 6, or 8
- Rhombohedral: CN = 3, 4, or 6
- Monoclinic: CN = 4, 6, or 8
- Triclinic: CN = 4, 6, or 8
- Hexagonal: CN = 6
**6. Packing Efficiency**
- Packing efficiency = (Volume occupied by spheres) / (Total volume of unit cell)
- Cubic (primitive) packing efficiency = π / 6 ≈ 0.52
- Cubic (body-centered) packing efficiency = π / 3 ≈ 0.68
- Cubic (face-centered) packing efficiency = π / 2 ≈ 0.74
- Hexagonal packing efficiency = 0.74
**7. X-Ray Diffraction**
- Bragg's law: nλ = 2d sinθ, where n is the order of the diffraction, λ is the wavelength of X-rays, d is the distance between atomic planes, and θ is the angle of incidence.
**8. Electrical Conductivity**
- Conductors: High electrical conductivity due to the presence of a large number of free electrons.
- Insulators: Poor electrical conductivity due to a completely filled valence band and large energy gap.
- Semiconductors: Moderate electrical conductivity due to a partially filled valence band and a small energy gap.
These are some of the key formulas and concepts that you should include in your Solid State formula sheet for NEET preparation. Regular revision and practice using these formulas will enhance your understanding and problem
Attention NEET Students!
To make sure you are not studying endlessly, EduRev has designed NEET study material, with Structured Courses, Videos, & Test Series. Plus get personalized analysis, doubt solving and improvement plans to achieve a great score in NEET.

|
Explore Courses for NEET exam
|

|
Similar NEET Doubts
NEET PREPARATION SERIES (Formula sheet for Solid State)?
Question Description
NEET PREPARATION SERIES (Formula sheet for Solid State)? for NEET 2025 is part of NEET preparation. The Question and answers have been prepared according to the NEET exam syllabus. Information about NEET PREPARATION SERIES (Formula sheet for Solid State)? covers all topics & solutions for NEET 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for NEET PREPARATION SERIES (Formula sheet for Solid State)?.
NEET PREPARATION SERIES (Formula sheet for Solid State)? for NEET 2025 is part of NEET preparation. The Question and answers have been prepared according to the NEET exam syllabus. Information about NEET PREPARATION SERIES (Formula sheet for Solid State)? covers all topics & solutions for NEET 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for NEET PREPARATION SERIES (Formula sheet for Solid State)?.
Solutions for NEET PREPARATION SERIES (Formula sheet for Solid State)? in English & in Hindi are available as part of our courses for NEET.
Download more important topics, notes, lectures and mock test series for NEET Exam by signing up for free.
Here you can find the meaning of NEET PREPARATION SERIES (Formula sheet for Solid State)? defined & explained in the simplest way possible. Besides giving the explanation of
NEET PREPARATION SERIES (Formula sheet for Solid State)?, a detailed solution for NEET PREPARATION SERIES (Formula sheet for Solid State)? has been provided alongside types of NEET PREPARATION SERIES (Formula sheet for Solid State)? theory, EduRev gives you an
ample number of questions to practice NEET PREPARATION SERIES (Formula sheet for Solid State)? tests, examples and also practice NEET tests.

|
Explore Courses for NEET exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.

























