Class 10 Exam > Class 10 Questions > What is the easiest way to distinguish covale...
Start Learning for Free
What is the easiest way to distinguish covalent bond from ionic bond?
Most Upvoted Answer
What is the easiest way to distinguish covalent bond from ionic bond?
Community Answer
What is the easiest way to distinguish covalent bond from ionic bond?
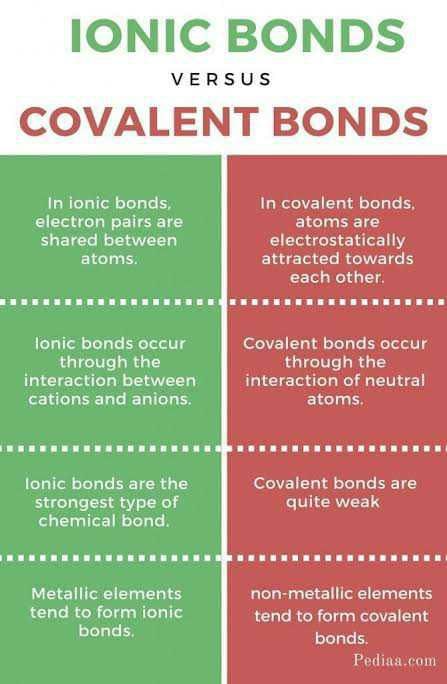
Covalent Bond vs Ionic Bond
There are some key differences between covalent and ionic bonds that can help you distinguish between the two types of chemical bonds. Here's a detailed explanation:
Covalent Bond
- In a covalent bond, atoms share electrons to achieve a stable electron configuration.
- Covalent bonds usually occur between non-metal atoms.
- The sharing of electrons in a covalent bond creates a strong bond that holds the atoms together.
- Covalent bonds are characterized by the formation of molecules.
- Covalent compounds typically have lower melting and boiling points compared to ionic compounds.
- Examples of compounds with covalent bonds include water (H2O), methane (CH4), and carbon dioxide (CO2).
Ionic Bond
- In an ionic bond, electrons are transferred from one atom to another to achieve a stable electron configuration.
- Ionic bonds usually occur between a metal and a non-metal atom.
- The transfer of electrons in an ionic bond results in the formation of ions with opposite charges that are held together by electrostatic forces.
- Ionic bonds are characterized by the formation of ionic compounds, also known as salts.
- Ionic compounds typically have higher melting and boiling points compared to covalent compounds.
- Examples of compounds with ionic bonds include sodium chloride (NaCl), magnesium oxide (MgO), and calcium carbonate (CaCO3).
By understanding these key differences, you can easily distinguish between covalent and ionic bonds based on the nature of the bond, the types of atoms involved, the formation of molecules or ions, and the physical properties of the compounds.
There are some key differences between covalent and ionic bonds that can help you distinguish between the two types of chemical bonds. Here's a detailed explanation:
Covalent Bond
- In a covalent bond, atoms share electrons to achieve a stable electron configuration.
- Covalent bonds usually occur between non-metal atoms.
- The sharing of electrons in a covalent bond creates a strong bond that holds the atoms together.
- Covalent bonds are characterized by the formation of molecules.
- Covalent compounds typically have lower melting and boiling points compared to ionic compounds.
- Examples of compounds with covalent bonds include water (H2O), methane (CH4), and carbon dioxide (CO2).
Ionic Bond
- In an ionic bond, electrons are transferred from one atom to another to achieve a stable electron configuration.
- Ionic bonds usually occur between a metal and a non-metal atom.
- The transfer of electrons in an ionic bond results in the formation of ions with opposite charges that are held together by electrostatic forces.
- Ionic bonds are characterized by the formation of ionic compounds, also known as salts.
- Ionic compounds typically have higher melting and boiling points compared to covalent compounds.
- Examples of compounds with ionic bonds include sodium chloride (NaCl), magnesium oxide (MgO), and calcium carbonate (CaCO3).
By understanding these key differences, you can easily distinguish between covalent and ionic bonds based on the nature of the bond, the types of atoms involved, the formation of molecules or ions, and the physical properties of the compounds.

|
Explore Courses for Class 10 exam
|

|
Similar Class 10 Doubts
What is the easiest way to distinguish covalent bond from ionic bond?
Question Description
What is the easiest way to distinguish covalent bond from ionic bond? for Class 10 2025 is part of Class 10 preparation. The Question and answers have been prepared according to the Class 10 exam syllabus. Information about What is the easiest way to distinguish covalent bond from ionic bond? covers all topics & solutions for Class 10 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for What is the easiest way to distinguish covalent bond from ionic bond?.
What is the easiest way to distinguish covalent bond from ionic bond? for Class 10 2025 is part of Class 10 preparation. The Question and answers have been prepared according to the Class 10 exam syllabus. Information about What is the easiest way to distinguish covalent bond from ionic bond? covers all topics & solutions for Class 10 2025 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for What is the easiest way to distinguish covalent bond from ionic bond?.
Solutions for What is the easiest way to distinguish covalent bond from ionic bond? in English & in Hindi are available as part of our courses for Class 10.
Download more important topics, notes, lectures and mock test series for Class 10 Exam by signing up for free.
Here you can find the meaning of What is the easiest way to distinguish covalent bond from ionic bond? defined & explained in the simplest way possible. Besides giving the explanation of
What is the easiest way to distinguish covalent bond from ionic bond?, a detailed solution for What is the easiest way to distinguish covalent bond from ionic bond? has been provided alongside types of What is the easiest way to distinguish covalent bond from ionic bond? theory, EduRev gives you an
ample number of questions to practice What is the easiest way to distinguish covalent bond from ionic bond? tests, examples and also practice Class 10 tests.

|
Explore Courses for Class 10 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


























