NEET Exam > NEET Questions > How phenol is prepared from chlorobenzene.?
Start Learning for Free
How phenol is prepared from chlorobenzene.?
Most Upvoted Answer
How phenol is prepared from chlorobenzene.?
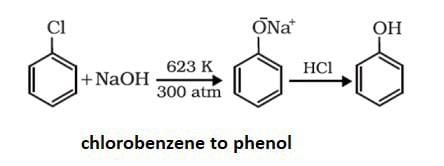
Community Answer
How phenol is prepared from chlorobenzene.?
Preparation of Phenol from Chlorobenzene
Phenol can be prepared from chlorobenzene through a series of chemical reactions. The process involves the following steps:
1. Nitration of Chlorobenzene:
Chlorobenzene is first treated with a mixture of concentrated nitric acid (HNO3) and concentrated sulfuric acid (H2SO4) to undergo nitration. Nitration is the process of introducing a nitro group (-NO2) into an organic compound. In this reaction, one of the hydrogen atoms in chlorobenzene is replaced by the nitro group, resulting in the formation of nitrochlorobenzene.
2. Reduction of Nitrochlorobenzene:
The nitrochlorobenzene obtained from the nitration step is then reduced to aminochlorobenzene. This reduction reaction is typically carried out using a reducing agent such as iron (Fe) and hydrochloric acid (HCl). The nitro group is converted into an amino group (-NH2) in this process.
3. Conversion to Diazonium Salt:
Aminochlorobenzene is then converted into a diazonium salt by reacting it with sodium nitrite (NaNO2) and hydrochloric acid (HCl). This reaction is known as diazotization. In this step, the amino group is replaced by a diazonium group (-N2+Cl-) to form the diazonium salt.
4. Coupling Reaction:
The diazonium salt is then subjected to a coupling reaction with water, phenol, or its derivatives to yield phenol. The most common method is the Sandmeyer reaction, where the diazonium salt reacts with water to form phenol. This reaction is generally carried out at low temperatures and under acidic conditions.
5. Isolation and Purification:
After the formation of phenol, it is isolated and purified using various techniques such as distillation, extraction, and crystallization. These processes help remove impurities and obtain high-quality phenol.
Overall Reaction:
The overall reaction can be represented as follows:
Chlorobenzene + HNO3 + H2SO4 → Nitrochlorobenzene
Nitrochlorobenzene + Fe + HCl → Aminochlorobenzene
Aminochlorobenzene + NaNO2 + HCl → Diazonium Salt
Diazonium Salt + H2O → Phenol
Note:
It is important to follow proper safety precautions and handle the chemicals involved in these reactions with care, as they can be hazardous. It is recommended to perform these reactions under controlled laboratory conditions.
Phenol can be prepared from chlorobenzene through a series of chemical reactions. The process involves the following steps:
1. Nitration of Chlorobenzene:
Chlorobenzene is first treated with a mixture of concentrated nitric acid (HNO3) and concentrated sulfuric acid (H2SO4) to undergo nitration. Nitration is the process of introducing a nitro group (-NO2) into an organic compound. In this reaction, one of the hydrogen atoms in chlorobenzene is replaced by the nitro group, resulting in the formation of nitrochlorobenzene.
2. Reduction of Nitrochlorobenzene:
The nitrochlorobenzene obtained from the nitration step is then reduced to aminochlorobenzene. This reduction reaction is typically carried out using a reducing agent such as iron (Fe) and hydrochloric acid (HCl). The nitro group is converted into an amino group (-NH2) in this process.
3. Conversion to Diazonium Salt:
Aminochlorobenzene is then converted into a diazonium salt by reacting it with sodium nitrite (NaNO2) and hydrochloric acid (HCl). This reaction is known as diazotization. In this step, the amino group is replaced by a diazonium group (-N2+Cl-) to form the diazonium salt.
4. Coupling Reaction:
The diazonium salt is then subjected to a coupling reaction with water, phenol, or its derivatives to yield phenol. The most common method is the Sandmeyer reaction, where the diazonium salt reacts with water to form phenol. This reaction is generally carried out at low temperatures and under acidic conditions.
5. Isolation and Purification:
After the formation of phenol, it is isolated and purified using various techniques such as distillation, extraction, and crystallization. These processes help remove impurities and obtain high-quality phenol.
Overall Reaction:
The overall reaction can be represented as follows:
Chlorobenzene + HNO3 + H2SO4 → Nitrochlorobenzene
Nitrochlorobenzene + Fe + HCl → Aminochlorobenzene
Aminochlorobenzene + NaNO2 + HCl → Diazonium Salt
Diazonium Salt + H2O → Phenol
Note:
It is important to follow proper safety precautions and handle the chemicals involved in these reactions with care, as they can be hazardous. It is recommended to perform these reactions under controlled laboratory conditions.
Attention NEET Students!
To make sure you are not studying endlessly, EduRev has designed NEET study material, with Structured Courses, Videos, & Test Series. Plus get personalized analysis, doubt solving and improvement plans to achieve a great score in NEET.

|
Explore Courses for NEET exam
|

|
Similar NEET Doubts
How phenol is prepared from chlorobenzene.?
Question Description
How phenol is prepared from chlorobenzene.? for NEET 2024 is part of NEET preparation. The Question and answers have been prepared according to the NEET exam syllabus. Information about How phenol is prepared from chlorobenzene.? covers all topics & solutions for NEET 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for How phenol is prepared from chlorobenzene.?.
How phenol is prepared from chlorobenzene.? for NEET 2024 is part of NEET preparation. The Question and answers have been prepared according to the NEET exam syllabus. Information about How phenol is prepared from chlorobenzene.? covers all topics & solutions for NEET 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for How phenol is prepared from chlorobenzene.?.
Solutions for How phenol is prepared from chlorobenzene.? in English & in Hindi are available as part of our courses for NEET.
Download more important topics, notes, lectures and mock test series for NEET Exam by signing up for free.
Here you can find the meaning of How phenol is prepared from chlorobenzene.? defined & explained in the simplest way possible. Besides giving the explanation of
How phenol is prepared from chlorobenzene.?, a detailed solution for How phenol is prepared from chlorobenzene.? has been provided alongside types of How phenol is prepared from chlorobenzene.? theory, EduRev gives you an
ample number of questions to practice How phenol is prepared from chlorobenzene.? tests, examples and also practice NEET tests.

|
Explore Courses for NEET exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.

























