SSC Exam > SSC Questions > Stirling and Ericsson cycle area)Reversible ...
Start Learning for Free
Stirling and Ericsson cycle are
- a)Reversible cycles
- b)Irreversible cycles
- c)Quasi-static cycles
- d)Semi-reversible cycle
Correct answer is option 'A'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
Stirling and Ericsson cycle area)Reversible cyclesb)Irreversible cycl...
Stirling cycle and Ericsson cycle are the modified forms of the Carnot cycle.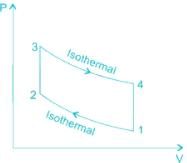
View all questions of this test
A Stirling cycle consists of two reversible isothermal and two reversible constant volume (isochoric) processes.


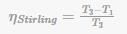
ηStirling = T3/T1/T3
Which is the same as Carnot’s efficiency.
Whereas an Ericsson cycle consists of two reversible isothermal and two reversible constant pressure (isobaric) processes.
ηericsson = 1−T1/T3which is the same as Carnot efficiency
Most Upvoted Answer
Stirling and Ericsson cycle area)Reversible cyclesb)Irreversible cycl...
Reversible cycles:
A reversible cycle is a theoretical concept in thermodynamics where the system undergoes a series of processes that can be reversed by an infinitesimal change in any of the parameters. In a reversible cycle, the system always remains in equilibrium with its surroundings throughout the entire process. This means that both the forward and backward processes can be executed without any loss of energy or increase in entropy.
Irreversible cycles:
On the other hand, an irreversible cycle is a real-world process that deviates from the ideal conditions of reversibility. In an irreversible cycle, there is always some loss of energy due to various factors such as friction, heat transfer across finite temperature differences, and irreversibilities within the system. These losses result in an increase in entropy and a decrease in the efficiency of the cycle compared to a reversible cycle.
Quasi-static cycles:
A quasi-static cycle is a concept used in thermodynamics to describe a process that occurs at an infinitesimally slow rate. In a quasi-static cycle, the system is always in equilibrium with its surroundings, and the deviation from equilibrium is minimized. This allows the system to follow a well-defined path and ensures that the properties of the system can be determined at any point during the process.
Semi-reversible cycles:
A semi-reversible cycle is a combination of reversible and irreversible processes. In a semi-reversible cycle, certain processes may be reversible while others may be irreversible. This type of cycle is often used to model real-world systems where some processes can be approximated as reversible while others cannot.
Correct answer:
The correct answer to the given question is option 'A', which states that the Stirling and Ericsson cycles are reversible cycles. This means that these cycles can be executed in both the forward and backward directions without any loss of energy or increase in entropy. The Stirling and Ericsson cycles are often used as idealized models for certain heat engines and refrigeration systems.
A reversible cycle is a theoretical concept in thermodynamics where the system undergoes a series of processes that can be reversed by an infinitesimal change in any of the parameters. In a reversible cycle, the system always remains in equilibrium with its surroundings throughout the entire process. This means that both the forward and backward processes can be executed without any loss of energy or increase in entropy.
Irreversible cycles:
On the other hand, an irreversible cycle is a real-world process that deviates from the ideal conditions of reversibility. In an irreversible cycle, there is always some loss of energy due to various factors such as friction, heat transfer across finite temperature differences, and irreversibilities within the system. These losses result in an increase in entropy and a decrease in the efficiency of the cycle compared to a reversible cycle.
Quasi-static cycles:
A quasi-static cycle is a concept used in thermodynamics to describe a process that occurs at an infinitesimally slow rate. In a quasi-static cycle, the system is always in equilibrium with its surroundings, and the deviation from equilibrium is minimized. This allows the system to follow a well-defined path and ensures that the properties of the system can be determined at any point during the process.
Semi-reversible cycles:
A semi-reversible cycle is a combination of reversible and irreversible processes. In a semi-reversible cycle, certain processes may be reversible while others may be irreversible. This type of cycle is often used to model real-world systems where some processes can be approximated as reversible while others cannot.
Correct answer:
The correct answer to the given question is option 'A', which states that the Stirling and Ericsson cycles are reversible cycles. This means that these cycles can be executed in both the forward and backward directions without any loss of energy or increase in entropy. The Stirling and Ericsson cycles are often used as idealized models for certain heat engines and refrigeration systems.

|
Explore Courses for SSC exam
|

|
Similar SSC Doubts
Stirling and Ericsson cycle area)Reversible cyclesb)Irreversible cyclesc)Quasi-static cyclesd)Semi-reversible cycleCorrect answer is option 'A'. Can you explain this answer?
Question Description
Stirling and Ericsson cycle area)Reversible cyclesb)Irreversible cyclesc)Quasi-static cyclesd)Semi-reversible cycleCorrect answer is option 'A'. Can you explain this answer? for SSC 2024 is part of SSC preparation. The Question and answers have been prepared according to the SSC exam syllabus. Information about Stirling and Ericsson cycle area)Reversible cyclesb)Irreversible cyclesc)Quasi-static cyclesd)Semi-reversible cycleCorrect answer is option 'A'. Can you explain this answer? covers all topics & solutions for SSC 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Stirling and Ericsson cycle area)Reversible cyclesb)Irreversible cyclesc)Quasi-static cyclesd)Semi-reversible cycleCorrect answer is option 'A'. Can you explain this answer?.
Stirling and Ericsson cycle area)Reversible cyclesb)Irreversible cyclesc)Quasi-static cyclesd)Semi-reversible cycleCorrect answer is option 'A'. Can you explain this answer? for SSC 2024 is part of SSC preparation. The Question and answers have been prepared according to the SSC exam syllabus. Information about Stirling and Ericsson cycle area)Reversible cyclesb)Irreversible cyclesc)Quasi-static cyclesd)Semi-reversible cycleCorrect answer is option 'A'. Can you explain this answer? covers all topics & solutions for SSC 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Stirling and Ericsson cycle area)Reversible cyclesb)Irreversible cyclesc)Quasi-static cyclesd)Semi-reversible cycleCorrect answer is option 'A'. Can you explain this answer?.
Solutions for Stirling and Ericsson cycle area)Reversible cyclesb)Irreversible cyclesc)Quasi-static cyclesd)Semi-reversible cycleCorrect answer is option 'A'. Can you explain this answer? in English & in Hindi are available as part of our courses for SSC.
Download more important topics, notes, lectures and mock test series for SSC Exam by signing up for free.
Here you can find the meaning of Stirling and Ericsson cycle area)Reversible cyclesb)Irreversible cyclesc)Quasi-static cyclesd)Semi-reversible cycleCorrect answer is option 'A'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Stirling and Ericsson cycle area)Reversible cyclesb)Irreversible cyclesc)Quasi-static cyclesd)Semi-reversible cycleCorrect answer is option 'A'. Can you explain this answer?, a detailed solution for Stirling and Ericsson cycle area)Reversible cyclesb)Irreversible cyclesc)Quasi-static cyclesd)Semi-reversible cycleCorrect answer is option 'A'. Can you explain this answer? has been provided alongside types of Stirling and Ericsson cycle area)Reversible cyclesb)Irreversible cyclesc)Quasi-static cyclesd)Semi-reversible cycleCorrect answer is option 'A'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Stirling and Ericsson cycle area)Reversible cyclesb)Irreversible cyclesc)Quasi-static cyclesd)Semi-reversible cycleCorrect answer is option 'A'. Can you explain this answer? tests, examples and also practice SSC tests.

|
Explore Courses for SSC exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.


















