Class 10 Exam > Class 10 Questions > If a sample of water containing detergents i...
Start Learning for Free
If a sample of water containing detergents is provided to you, which of the following methods will you adopt to neutralize it?
- a)Treating the water with baking soda
- b)Treating the water with vinegar
- c)Treating the water with caustic soda
- d)Treating the water with washing soda
Correct answer is option 'B'. Can you explain this answer?
| FREE This question is part of | Download PDF Attempt this Test |
Verified Answer
If a sample of water containing detergents is provided to you, which ...
Neutralization takes place when Acid and Base react to form Salt and Water
View all questions of this test
Acid + Base → Salt + Water
Detergent is a base and Vinegar is an acid, thus neutralisation takes place.Neutralization Reaction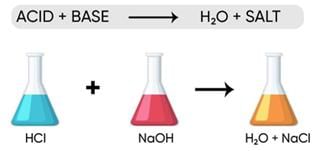

Most Upvoted Answer
If a sample of water containing detergents is provided to you, which ...
Method to Neutralize Water Containing Detergents
To neutralize water containing detergents, the most suitable method is to treat the water with vinegar. Vinegar, which is a weak acid, can effectively neutralize the alkaline detergents present in the water. Let's explore this method in detail:
1. Understanding the Problem:
When water contains detergents, it tends to become alkaline due to the presence of alkaline substances in the detergent. This can lead to several issues such as skin irritation, damage to aquatic life, and interference with the natural pH balance of the water.
2. Acid-Base Neutralization:
Neutralization is a chemical reaction that occurs between an acid and a base, resulting in the formation of water and a salt. In this case, we need to neutralize the alkaline detergents present in the water. Vinegar, which contains acetic acid, can act as an acid to neutralize the alkalinity.
3. Treating the Water with Vinegar:
To neutralize the water containing detergents, the following steps can be followed:
- Take a sample of the water containing detergents in a container.
- Gradually add vinegar to the water, stirring it gently.
- Continue adding vinegar until the pH of the water reaches a neutral level, which is around 7 on the pH scale.
- Regularly test the pH of the water using a pH testing kit or pH meter until it reaches the desired neutral level.
4. Benefits of Using Vinegar:
Treating the water with vinegar offers several benefits:
- Vinegar is readily available and affordable, making it easily accessible for neutralization purposes.
- Vinegar is a natural and environmentally friendly solution, minimizing the impact on the ecosystem.
- Vinegar is a weak acid, so it does not pose a significant risk of harm to individuals handling it.
- Vinegar effectively neutralizes the alkaline detergents, restoring the water's pH balance.
5. Precautions:
While treating the water with vinegar, it is important to keep the following precautions in mind:
- Use vinegar in appropriate quantities to avoid over-neutralizing the water or introducing excess acidity.
- Handle vinegar with care to prevent any accidental spills or contact with eyes or skin.
- Dispose of the neutralized water properly, taking into consideration any local regulations or guidelines for wastewater treatment.
By following these steps and using vinegar as a neutralizing agent, the water containing detergents can be effectively neutralized, ensuring its safety for various purposes.
To neutralize water containing detergents, the most suitable method is to treat the water with vinegar. Vinegar, which is a weak acid, can effectively neutralize the alkaline detergents present in the water. Let's explore this method in detail:
1. Understanding the Problem:
When water contains detergents, it tends to become alkaline due to the presence of alkaline substances in the detergent. This can lead to several issues such as skin irritation, damage to aquatic life, and interference with the natural pH balance of the water.
2. Acid-Base Neutralization:
Neutralization is a chemical reaction that occurs between an acid and a base, resulting in the formation of water and a salt. In this case, we need to neutralize the alkaline detergents present in the water. Vinegar, which contains acetic acid, can act as an acid to neutralize the alkalinity.
3. Treating the Water with Vinegar:
To neutralize the water containing detergents, the following steps can be followed:
- Take a sample of the water containing detergents in a container.
- Gradually add vinegar to the water, stirring it gently.
- Continue adding vinegar until the pH of the water reaches a neutral level, which is around 7 on the pH scale.
- Regularly test the pH of the water using a pH testing kit or pH meter until it reaches the desired neutral level.
4. Benefits of Using Vinegar:
Treating the water with vinegar offers several benefits:
- Vinegar is readily available and affordable, making it easily accessible for neutralization purposes.
- Vinegar is a natural and environmentally friendly solution, minimizing the impact on the ecosystem.
- Vinegar is a weak acid, so it does not pose a significant risk of harm to individuals handling it.
- Vinegar effectively neutralizes the alkaline detergents, restoring the water's pH balance.
5. Precautions:
While treating the water with vinegar, it is important to keep the following precautions in mind:
- Use vinegar in appropriate quantities to avoid over-neutralizing the water or introducing excess acidity.
- Handle vinegar with care to prevent any accidental spills or contact with eyes or skin.
- Dispose of the neutralized water properly, taking into consideration any local regulations or guidelines for wastewater treatment.
By following these steps and using vinegar as a neutralizing agent, the water containing detergents can be effectively neutralized, ensuring its safety for various purposes.
Attention Class 10 Students!
To make sure you are not studying endlessly, EduRev has designed Class 10 study material, with Structured Courses, Videos, & Test Series. Plus get personalized analysis, doubt solving and improvement plans to achieve a great score in Class 10.

|
Explore Courses for Class 10 exam
|

|
Similar Class 10 Doubts
If a sample of water containing detergents is provided to you, which of the following methods will you adopt to neutralize it?a)Treating the water with baking sodab)Treating the water with vinegarc)Treating the water with caustic sodad)Treating the water with washing sodaCorrect answer is option 'B'. Can you explain this answer?
Question Description
If a sample of water containing detergents is provided to you, which of the following methods will you adopt to neutralize it?a)Treating the water with baking sodab)Treating the water with vinegarc)Treating the water with caustic sodad)Treating the water with washing sodaCorrect answer is option 'B'. Can you explain this answer? for Class 10 2024 is part of Class 10 preparation. The Question and answers have been prepared according to the Class 10 exam syllabus. Information about If a sample of water containing detergents is provided to you, which of the following methods will you adopt to neutralize it?a)Treating the water with baking sodab)Treating the water with vinegarc)Treating the water with caustic sodad)Treating the water with washing sodaCorrect answer is option 'B'. Can you explain this answer? covers all topics & solutions for Class 10 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for If a sample of water containing detergents is provided to you, which of the following methods will you adopt to neutralize it?a)Treating the water with baking sodab)Treating the water with vinegarc)Treating the water with caustic sodad)Treating the water with washing sodaCorrect answer is option 'B'. Can you explain this answer?.
If a sample of water containing detergents is provided to you, which of the following methods will you adopt to neutralize it?a)Treating the water with baking sodab)Treating the water with vinegarc)Treating the water with caustic sodad)Treating the water with washing sodaCorrect answer is option 'B'. Can you explain this answer? for Class 10 2024 is part of Class 10 preparation. The Question and answers have been prepared according to the Class 10 exam syllabus. Information about If a sample of water containing detergents is provided to you, which of the following methods will you adopt to neutralize it?a)Treating the water with baking sodab)Treating the water with vinegarc)Treating the water with caustic sodad)Treating the water with washing sodaCorrect answer is option 'B'. Can you explain this answer? covers all topics & solutions for Class 10 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for If a sample of water containing detergents is provided to you, which of the following methods will you adopt to neutralize it?a)Treating the water with baking sodab)Treating the water with vinegarc)Treating the water with caustic sodad)Treating the water with washing sodaCorrect answer is option 'B'. Can you explain this answer?.
Solutions for If a sample of water containing detergents is provided to you, which of the following methods will you adopt to neutralize it?a)Treating the water with baking sodab)Treating the water with vinegarc)Treating the water with caustic sodad)Treating the water with washing sodaCorrect answer is option 'B'. Can you explain this answer? in English & in Hindi are available as part of our courses for Class 10.
Download more important topics, notes, lectures and mock test series for Class 10 Exam by signing up for free.
Here you can find the meaning of If a sample of water containing detergents is provided to you, which of the following methods will you adopt to neutralize it?a)Treating the water with baking sodab)Treating the water with vinegarc)Treating the water with caustic sodad)Treating the water with washing sodaCorrect answer is option 'B'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
If a sample of water containing detergents is provided to you, which of the following methods will you adopt to neutralize it?a)Treating the water with baking sodab)Treating the water with vinegarc)Treating the water with caustic sodad)Treating the water with washing sodaCorrect answer is option 'B'. Can you explain this answer?, a detailed solution for If a sample of water containing detergents is provided to you, which of the following methods will you adopt to neutralize it?a)Treating the water with baking sodab)Treating the water with vinegarc)Treating the water with caustic sodad)Treating the water with washing sodaCorrect answer is option 'B'. Can you explain this answer? has been provided alongside types of If a sample of water containing detergents is provided to you, which of the following methods will you adopt to neutralize it?a)Treating the water with baking sodab)Treating the water with vinegarc)Treating the water with caustic sodad)Treating the water with washing sodaCorrect answer is option 'B'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice If a sample of water containing detergents is provided to you, which of the following methods will you adopt to neutralize it?a)Treating the water with baking sodab)Treating the water with vinegarc)Treating the water with caustic sodad)Treating the water with washing sodaCorrect answer is option 'B'. Can you explain this answer? tests, examples and also practice Class 10 tests.

|
Explore Courses for Class 10 exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
























