Class 10 Exam > Class 10 Questions > Assertion : To dilute the concentrated sulphu...
Start Learning for Free
Assertion : To dilute the concentrated sulphuric acid, water is added to the acid slowly.
Reason : A lot of heat energy will be given out in the dilution of concentrated sulphuric acid.
- a).Assertion (A) is false but reason (R) is true.
- b)Both assertion (A) and reason (R) are true but reason (R) is not the correct explanation of assertion (A).
- c)Assertion (A) is true but reason (R) is false.
- d)Both assertion (A) and reason (R) are true and reason (R) is the correct explanation of assertion (A)
- e)Both Assertion and Reason are false.
Correct answer is option 'A'. Can you explain this answer?
Verified Answer
Assertion : To dilute the concentrated sulphuric acid, water is added ...
Assertion (A): To dilute concentrated sulphuric acid, water is added to the acid slowly.
- This statement is true. Concentrated sulfuric acid is highly exothermic when mixed with water. Adding water to concentrated sulfuric acid slowly helps to control the reaction and minimize the risk of splashing or violent boiling due to the large amount of heat released.
Reason (R): A lot of heat energy will be given out in the dilution of concentrated sulphuric acid.
- This statement is true. Diluting concentrated sulfuric acid with water is highly exothermic. The reaction between sulfuric acid and water is:
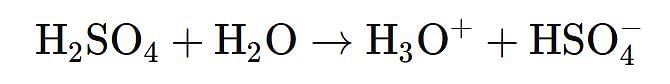
This reaction releases a large amount of heat energy, which can lead to rapid heating of the solution if not added slowly.
- This statement is true. Diluting concentrated sulfuric acid with water is highly exothermic. The reaction between sulfuric acid and water is:
 This question is part of UPSC exam. View all Class 10 courses
This question is part of UPSC exam. View all Class 10 courses
Most Upvoted Answer
Assertion : To dilute the concentrated sulphuric acid, water is added ...
To dilute concentrated sulphuric acid, water is added slowly. The assertion is that the dilution process requires the addition of water to the acid gradually, and the reason provided is that a lot of heat energy is released during the dilution of concentrated sulphuric acid.
Explanation:
The dilution of concentrated sulphuric acid is an exothermic process, meaning that it releases heat energy. This is due to the highly exothermic nature of the reaction between concentrated sulphuric acid and water. When water is added to the acid, the acid molecules dissociate into ions, and this process is highly exothermic. The heat released during this reaction can be intense and can cause the mixture to boil or splatter if not added carefully.
To avoid any accidents or hazards, it is important to add water to the acid slowly. By adding water gradually, the heat released can be dissipated more effectively, reducing the risk of splattering or boiling of the mixture. This allows for a safer dilution process.
Moreover, adding water to concentrated sulphuric acid too quickly can lead to a highly exothermic reaction and result in the sudden generation of steam. The rapid expansion of steam can cause the mixture to violently splatter, posing a significant risk of burns or other injuries.
Hence, the assertion that water should be added slowly to dilute concentrated sulphuric acid is true. The reason provided, that a lot of heat energy is released during the dilution process, is also true and supports the assertion. However, the reason does not directly explain why water should be added slowly. It simply highlights the importance of handling the heat energy released during the dilution process with caution. Therefore, option A is the correct answer.
Explanation:
The dilution of concentrated sulphuric acid is an exothermic process, meaning that it releases heat energy. This is due to the highly exothermic nature of the reaction between concentrated sulphuric acid and water. When water is added to the acid, the acid molecules dissociate into ions, and this process is highly exothermic. The heat released during this reaction can be intense and can cause the mixture to boil or splatter if not added carefully.
To avoid any accidents or hazards, it is important to add water to the acid slowly. By adding water gradually, the heat released can be dissipated more effectively, reducing the risk of splattering or boiling of the mixture. This allows for a safer dilution process.
Moreover, adding water to concentrated sulphuric acid too quickly can lead to a highly exothermic reaction and result in the sudden generation of steam. The rapid expansion of steam can cause the mixture to violently splatter, posing a significant risk of burns or other injuries.
Hence, the assertion that water should be added slowly to dilute concentrated sulphuric acid is true. The reason provided, that a lot of heat energy is released during the dilution process, is also true and supports the assertion. However, the reason does not directly explain why water should be added slowly. It simply highlights the importance of handling the heat energy released during the dilution process with caution. Therefore, option A is the correct answer.
Attention Class 10 Students!
To make sure you are not studying endlessly, EduRev has designed Class 10 study material, with Structured Courses, Videos, & Test Series. Plus get personalized analysis, doubt solving and improvement plans to achieve a great score in Class 10.

|
Explore Courses for Class 10 exam
|

|
Similar Class 10 Doubts
Assertion : To dilute the concentrated sulphuric acid, water is added to the acid slowly.Reason : A lot of heat energy will be given out in the dilution of concentrated sulphuric acid.a).Assertion (A) is false but reason (R) is true.b)Both assertion (A) and reason (R) are true but reason (R) is not the correct explanation of assertion (A).c)Assertion (A) is true but reason (R) is false.d)Both assertion (A) and reason (R) are true and reason (R) is the correct explanation of assertion (A)e)Both Assertion and Reason are false.Correct answer is option 'A'. Can you explain this answer?
Question Description
Assertion : To dilute the concentrated sulphuric acid, water is added to the acid slowly.Reason : A lot of heat energy will be given out in the dilution of concentrated sulphuric acid.a).Assertion (A) is false but reason (R) is true.b)Both assertion (A) and reason (R) are true but reason (R) is not the correct explanation of assertion (A).c)Assertion (A) is true but reason (R) is false.d)Both assertion (A) and reason (R) are true and reason (R) is the correct explanation of assertion (A)e)Both Assertion and Reason are false.Correct answer is option 'A'. Can you explain this answer? for Class 10 2024 is part of Class 10 preparation. The Question and answers have been prepared according to the Class 10 exam syllabus. Information about Assertion : To dilute the concentrated sulphuric acid, water is added to the acid slowly.Reason : A lot of heat energy will be given out in the dilution of concentrated sulphuric acid.a).Assertion (A) is false but reason (R) is true.b)Both assertion (A) and reason (R) are true but reason (R) is not the correct explanation of assertion (A).c)Assertion (A) is true but reason (R) is false.d)Both assertion (A) and reason (R) are true and reason (R) is the correct explanation of assertion (A)e)Both Assertion and Reason are false.Correct answer is option 'A'. Can you explain this answer? covers all topics & solutions for Class 10 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Assertion : To dilute the concentrated sulphuric acid, water is added to the acid slowly.Reason : A lot of heat energy will be given out in the dilution of concentrated sulphuric acid.a).Assertion (A) is false but reason (R) is true.b)Both assertion (A) and reason (R) are true but reason (R) is not the correct explanation of assertion (A).c)Assertion (A) is true but reason (R) is false.d)Both assertion (A) and reason (R) are true and reason (R) is the correct explanation of assertion (A)e)Both Assertion and Reason are false.Correct answer is option 'A'. Can you explain this answer?.
Assertion : To dilute the concentrated sulphuric acid, water is added to the acid slowly.Reason : A lot of heat energy will be given out in the dilution of concentrated sulphuric acid.a).Assertion (A) is false but reason (R) is true.b)Both assertion (A) and reason (R) are true but reason (R) is not the correct explanation of assertion (A).c)Assertion (A) is true but reason (R) is false.d)Both assertion (A) and reason (R) are true and reason (R) is the correct explanation of assertion (A)e)Both Assertion and Reason are false.Correct answer is option 'A'. Can you explain this answer? for Class 10 2024 is part of Class 10 preparation. The Question and answers have been prepared according to the Class 10 exam syllabus. Information about Assertion : To dilute the concentrated sulphuric acid, water is added to the acid slowly.Reason : A lot of heat energy will be given out in the dilution of concentrated sulphuric acid.a).Assertion (A) is false but reason (R) is true.b)Both assertion (A) and reason (R) are true but reason (R) is not the correct explanation of assertion (A).c)Assertion (A) is true but reason (R) is false.d)Both assertion (A) and reason (R) are true and reason (R) is the correct explanation of assertion (A)e)Both Assertion and Reason are false.Correct answer is option 'A'. Can you explain this answer? covers all topics & solutions for Class 10 2024 Exam. Find important definitions, questions, meanings, examples, exercises and tests below for Assertion : To dilute the concentrated sulphuric acid, water is added to the acid slowly.Reason : A lot of heat energy will be given out in the dilution of concentrated sulphuric acid.a).Assertion (A) is false but reason (R) is true.b)Both assertion (A) and reason (R) are true but reason (R) is not the correct explanation of assertion (A).c)Assertion (A) is true but reason (R) is false.d)Both assertion (A) and reason (R) are true and reason (R) is the correct explanation of assertion (A)e)Both Assertion and Reason are false.Correct answer is option 'A'. Can you explain this answer?.
Solutions for Assertion : To dilute the concentrated sulphuric acid, water is added to the acid slowly.Reason : A lot of heat energy will be given out in the dilution of concentrated sulphuric acid.a).Assertion (A) is false but reason (R) is true.b)Both assertion (A) and reason (R) are true but reason (R) is not the correct explanation of assertion (A).c)Assertion (A) is true but reason (R) is false.d)Both assertion (A) and reason (R) are true and reason (R) is the correct explanation of assertion (A)e)Both Assertion and Reason are false.Correct answer is option 'A'. Can you explain this answer? in English & in Hindi are available as part of our courses for Class 10.
Download more important topics, notes, lectures and mock test series for Class 10 Exam by signing up for free.
Here you can find the meaning of Assertion : To dilute the concentrated sulphuric acid, water is added to the acid slowly.Reason : A lot of heat energy will be given out in the dilution of concentrated sulphuric acid.a).Assertion (A) is false but reason (R) is true.b)Both assertion (A) and reason (R) are true but reason (R) is not the correct explanation of assertion (A).c)Assertion (A) is true but reason (R) is false.d)Both assertion (A) and reason (R) are true and reason (R) is the correct explanation of assertion (A)e)Both Assertion and Reason are false.Correct answer is option 'A'. Can you explain this answer? defined & explained in the simplest way possible. Besides giving the explanation of
Assertion : To dilute the concentrated sulphuric acid, water is added to the acid slowly.Reason : A lot of heat energy will be given out in the dilution of concentrated sulphuric acid.a).Assertion (A) is false but reason (R) is true.b)Both assertion (A) and reason (R) are true but reason (R) is not the correct explanation of assertion (A).c)Assertion (A) is true but reason (R) is false.d)Both assertion (A) and reason (R) are true and reason (R) is the correct explanation of assertion (A)e)Both Assertion and Reason are false.Correct answer is option 'A'. Can you explain this answer?, a detailed solution for Assertion : To dilute the concentrated sulphuric acid, water is added to the acid slowly.Reason : A lot of heat energy will be given out in the dilution of concentrated sulphuric acid.a).Assertion (A) is false but reason (R) is true.b)Both assertion (A) and reason (R) are true but reason (R) is not the correct explanation of assertion (A).c)Assertion (A) is true but reason (R) is false.d)Both assertion (A) and reason (R) are true and reason (R) is the correct explanation of assertion (A)e)Both Assertion and Reason are false.Correct answer is option 'A'. Can you explain this answer? has been provided alongside types of Assertion : To dilute the concentrated sulphuric acid, water is added to the acid slowly.Reason : A lot of heat energy will be given out in the dilution of concentrated sulphuric acid.a).Assertion (A) is false but reason (R) is true.b)Both assertion (A) and reason (R) are true but reason (R) is not the correct explanation of assertion (A).c)Assertion (A) is true but reason (R) is false.d)Both assertion (A) and reason (R) are true and reason (R) is the correct explanation of assertion (A)e)Both Assertion and Reason are false.Correct answer is option 'A'. Can you explain this answer? theory, EduRev gives you an
ample number of questions to practice Assertion : To dilute the concentrated sulphuric acid, water is added to the acid slowly.Reason : A lot of heat energy will be given out in the dilution of concentrated sulphuric acid.a).Assertion (A) is false but reason (R) is true.b)Both assertion (A) and reason (R) are true but reason (R) is not the correct explanation of assertion (A).c)Assertion (A) is true but reason (R) is false.d)Both assertion (A) and reason (R) are true and reason (R) is the correct explanation of assertion (A)e)Both Assertion and Reason are false.Correct answer is option 'A'. Can you explain this answer? tests, examples and also practice Class 10 tests.

|
Explore Courses for Class 10 exam
|

|
Suggested Free Tests
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.

























